Z8320
Harmless

Posts: 7
Registered: 13-12-2009
Member Is Offline
Mood: No Mood
|
|
Phosphorus via reduction of phosphoric acid with carbon in a microwave
The patent attached describes a process for reducing phosphoric acid with carbon to white phosphorus. The patent gives a laboratory scale example in
which a quartz test tube is charged with carbon and phosphoric acid and irradiated under conditions fairly similar to those in a conventional
microwave oven. An inert or reducing gas flow is required, but the temperature reached by the reaction mixture is only 540 °C.
It seems simpler and safer than some of the other methods I've heard such as the decomposition of phosphine into its elements at 375 degrees celsius
which has a significant explosion risk if your setup is shoddy or you haven't thought it through. I'll probably try this once I get around to getting
myself a tank of inert gas. The reaction possibly produces enough carbon monoxide and hydrogen that I don't need to worry about an external gas
supply but can instead rely on the evolution of these gases to protect the phosphorus from air.
Has anyone made any attempts like this?
Attachment: Phosphorus-Microwave.pdf (67kB)
This file has been downloaded 1666 times
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
have you tried it? i would need a quartz reactor and som nitrogen but i REALLY want to try this!
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
According to reports, conventional microwave ovens can often be used for such reactions.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Of course, finding a quartz test tube is another matter. Could potentially use borosilicate, but that would be pushing it (temp. resistance up to
something like 600 C)...
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Quote: Originally posted by elementcollector1  | | Of course, finding a quartz test tube is another matter. Could potentially use borosilicate, but that would be pushing it (temp. resistance up to
something like 600 C)... |
Finding a quartz tube isn't a problem. The price isn't either. This site sells them for 30$ or so, which isn't too bad. I wouldn't try borosilicate, 540 is around the annealing point, and when you consider
what you're doing - producing deadly white phosphorus and carbon monoxide at >500 degrees in a microwave - you really don't want to take any
shortcuts.
I have a quartz test tube, but not a suitable microwave...  Anyways, I have a
buttload of WP as well - so it's not very interesting to me. Anyways, I have a
buttload of WP as well - so it's not very interesting to me.
This just in: 95,5 % of the world population lives outside the USA
Please drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
although the challenge of getting it is more rewarding....are you selling any WP?
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Quote: Originally posted by elementcollector1  | | Of course, finding a quartz test tube is another matter. Could potentially use borosilicate, but that would be pushing it (temp. resistance up to
something like 600 C)... |
Electric heaters such as this one contain quartz tubes which host resistive heating wire.
Find a defunct heater and take it apart. Collect the tubes and the wires. Profit. 
|
|
|
cal
Hazard to Self
 
Posts: 88
Registered: 7-2-2012
Member Is Offline
Mood: No Mood
|
|
quartz tube
I have quartz tube pieces from 12 inches up to 36 inches.
Thought is an action, which when acted upon becomes work and sometimes art!
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
How does one close them at one end? I don't think my blowtorch can do quartz...
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
i think an inert gas is required for this reaction (non oxydasing environment ) and it would be convienient to carry the Phosphorus along to a cold
trap . so both end dont need to be sealed,
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
500 °C is just slightly beyond the temperature an ordinary stove top can achieve.
One factor that should not be neglected is that sodium salts have a way of fusing with glass (and likely fused quartz also) at higher temperatures.
One should never heat dry sodium phosphate in a glass flask over a flame, because salt will just fuse with the glass, forming a glassy fusion that
melts at a lower temperature, and this can cause a hole to melt in the bottom of the flask.
|
|
|
bahamuth
Hazard to Others
  
Posts: 384
Registered: 3-11-2009
Location: Norway
Member Is Offline
Mood: Under stimulated
|
|
Well I can tell you quartz won't hold up to phosphoric acid at 540 degrees C.
http://www.advaluetech.com/clear_fused_quartz_technical_info...
Phosphoric acid is actually used to dissolve quartz for trace element analysis.
Any sufficiently advanced technology is indistinguishable from magic.
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Be that as it may. There is a good chance a conventional microwave will do the trick.
At least, I have that impression. I might have gotten that impression via the Science Madness Archives.
At any rate, such microwave ovens can be obtained "used" in the U.S., at a Goodwill Store, generally in the $10 to $20 dollar range.
I realize some folks are located in other parts of the world, and local availability may vary.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I wonder how the phosphoric acid is able to attack the quartz?
Possibly form some sort of pyrolic acid with the silica?
something like: (HO)2Si(OPO3H2)
Can phosphoric acid attack regular glass also at elevated temperatures?
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
bahamuth
Hazard to Others
  
Posts: 384
Registered: 3-11-2009
Location: Norway
Member Is Offline
Mood: Under stimulated
|
|
Quote: Originally posted by AndersHoveland  |
I wonder how the phosphoric acid is able to attack the quartz?
Possibly form some sort of pyrolic acid with the silica?
something like: (HO)2Si(OPO3H2)
Can phosphoric acid attack regular glass also at elevated temperatures? |
I had hot phosphoric acid etch my ceramic tabletop stove, with the stove set to max (so around 250-350 °C).
It's a 1st generation ceramic tabletop stove so there are no markings but looks very much like the Schott Ceran brand glass, composition unknown but I
guess it's not far from borosilicate.
But I have been using Schott Boro 3.3 glassware to boil phosphoric acid in, but have yet to see the same beaker used repeatedly suffer any etch marks.
Any sufficiently advanced technology is indistinguishable from magic.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Found this warning for fused quartz:
| Quote: | Do not use hydrofluoric acid (or hot phosphoric acid).
Alkaline solutions attack slowly at room temperature , but much faster at elevated temperatures. |
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
damn! what else is microwave transparent and hot acid resistant? its going to be much harder than espected...
but thats what makes it so interesting and rewarding! 
[Edited on 13-3-2013 by neptunium]
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
This is most interesting. I was thinking of making magnesium silicate retort (MgOH + SiO2 = MgSiO2 etc.), which is supposed to be transparent on the
microwaves and has a melting point of 1900C and is regarded as having high resistance towards chemicals in high temps. Options are to use direct
heating with coke up to 1600C, or then applying the microwave.
The problem with the microwave seems to be the reaction energy. The patent cites that they were able to produce about 2 grams in 2 hours using 1kW of
microwave power. This is far off scale of what would be feasible even for amateur.
Essentially my concept is of magnesium silicate "bottle", which would have, probably solid fitted steel tubing retort which would be impregnated
underwater. This pot would be placed within microwave, or then into coke bed and fired up. The gases would be led into the water tank aside the
hopefully forming phosphorus, and the waste gases would be safely exhausted via meters long chimney up to the skies for the birds' and green partys'
trouble.
Though phosphine is denser than air, it should be destroyed in situ. This provocates the need to lead the exhaust gases via tubing and bubble them
through something. What will react with PH3? NaOH? Hypochlorites? Acids? Something that won't interfere with carbon monoxide, of course.
[Edited on 6-2-2014 by testimento]
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
i suppose you could fill the entire microwave with butane, it requires a whole lot of oxygen to burn just decently, and if it has just enough oxygen
it wont probably be able to knock the microwave door open
you can probably connect a hose to the inside of the microwave, then connect hose to butane lighter refiller and take a metal plate with a hole in and
press against the valve, in my country we have coins with perfect holes for this..
otherwise 'dolomite' with sulfuric acid should give a good amount of CO2, bfesser got 25kg dolomite approx to give 11kg CO2
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|

No, really, you shouldn't, at least if you like your house/life.
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
Why in devil should the mic be filled with butane? You can generate CO2 very easily, if you really need to fill it with anything.
I was thinking of something like this:
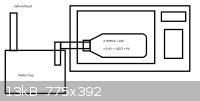
Just essentially a refractory ceramic (MgSiO is best afaik, ceramics are higly transparent to microwaves) bottle, most likely DIY, with steel tube
attached to the mouth and aligned down to water trap. The williepete will melt at 44C and evaporate at 280C and the reaction heat should be sufficient
to allow it to condense and flow into the tube, the vessel preferably aligned few degrees down so the liquid phossy will flow out. The reaction vessel
will be filled with copious amounts of CO and H2 and it will dispace any air present long before wp is produced.
Although this source cites that hot phossy acid is compatible with quartz:
http://www.sciencedirect.com/science/article/pii/S0168583X12...
So, if it is so, by far the easiest way is to just find quartz flask, put in the reactants and dump this into micro. 
http://fusedquartz.qsiquartz.com/viewitems/quartz-boiling-fl...
A suitable flask with joint seems to be in the 200usd price range. Jointing substantially simplifies the apparatus setup, since conventional lab
glassware can be used past the boiling phosphoric acid point.
For europeans, non-jointed model may be an option.
http://www.witeg.de/1749797951/1/PD61/8610/art/8610/0/1/61+P...
[Edited on 8-2-2014 by testimento]
[Edited on 8-2-2014 by testimento]
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
| Quote: |
500 °C is just slightly beyond the temperature an ordinary stove top can achieve. |
My hot plate can melt lead, zinc, and even aluminum once. So a least 660°C. My hot plate is not necessarily an 'ordinary' stovetop though.
[Edited on 8-2-2014 by Zyklonb]
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
All of my market bought hotplates can easily reach red hot temps. They're all in dumpyard at the moment though, since they failed - and I didn't even
mishandled them, but used them to heat conductively as they're supposed to. After wasting loads of money for that crap, I switched to propane burners
and had any problems. For high temp ops I use my oven which I've posted details on the other topic.
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
There's just one point regarding the patent cited in the first post. The amount of phosphorus produced per power of the microwave. With 1000 watts the
test shows only of 4.4 grams in two hours. This does not state the time curve of formation - does it all form within first 10 minutes or is the
production rate steady?
Because this means that one would need several kilowatts of magnetron power over very long period of time to produce even minimum amounts of
phosphorus with this method, thus if this is the case, rendering this method obsolete for most amateur chemists.
Unless the reaction could be hastened by heating the reaction vessel all the way up to 1000C...?
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
butane needs alot of oxygen to burn.. it can get somewhat violent when it reaches that amount however, but filling a bottle with butane and attempting
to light it will often result in a failure as you can very easily add too much butane
|
|
|