krfkeith
Harmless

Posts: 24
Registered: 17-4-2012
Location: Utah, United States
Member Is Offline
Mood: No Mood
|
|
Looking for uranium salts at non-insane prices, preferably from depleted feed-stock.
Sigh, uranium: such a useful element, and yet so hard to find in any form useful for the amateur chemist.
I've been wanting to play with some uranium related projects for awhile now. In particular, I'm in interested in trying to make some uranotype prints
(as the name suggests, it is a non-silver process which uses uranium) as well as some uranium/vaseline glass. The former mostly uses, I think, uranyl
nitrate. Whereas, according to to the few sources I could find on the subject, uranium glass was generally colored with approx. 1.5% by weight sodium
diuranate. I'm more than aware of the dangers of these chemicals, and I know to treat them with respect and caution. I am in the process of
constructing an electric furnace to melt rather small quantities of silica + other components in glass, and seeing as how glass melts already let of
some pretty nasty fumes, I'm working to make sure it has proper venting to get rid of these. As such, I don't think the compounds would post any real
hazard if handled correctly and generally not being stupid (i.e. not eating them).
Anyhow, the reason I mentioned depleted is that supposedly DU is something like 60% less radioactive than natural uranium. According to Theo Grey,
some the uranium glass he has (specifically stuff made before depleted uranium was even being made) shows non negligible levels of radiation compared
to similar items made with DU. The problem I have is two-fold. First of all, depleted or not, all of the sources I have found for uranium are insanely
priced. Ore, while still expensive, seems to be the cheapest, but purifying the uranium from this seems like an unnecessarily difficult undertaking.
There are more pure, reagent grade products that I have come across, but they are off-the-charts pricey. 2spi.com is the only source I have found that
is unequivocally DU, but they want something like absurd like $70 for a single gram of uranyl nitrate. Not nearly enough to be useful for either of my
projects. Ebay fared a little better, I found 10g of uranyl nitrate for $35. However, I am not sure if it is depleted (maybe that doesn't even matter,
who knows), I don't know how reliable the source is if I want to do more of this for the future, and it's still a little pricey (but not out of
sight).
If anyone here has any information on this subject, I would much appreciate it.
|
|
|
dontasker
Harmless

Posts: 40
Registered: 19-12-2012
Member Is Offline
Mood: Plopping
|
|
I've been occasionally looking for similar stuff.
The thing that ruins any attempt of finding a source is that if you do any searches for obtaining some you end up with a Yahoo Questions with some
twelve year old asking where he can buy some followed by some 90 people all frothing from the mouth about how even looking at uranium will make you
die.
You also get pictures of babies with harlequin ichthyosis and how that is proof of the dangers of DU ammo.
Depleted Uranium is dangerous, but it's not radioactive enough to warrant all the freaking out over that aspect of it. It can for some very toxic
salts, but chances are you will encounter far worse long before you get to the point of attempting experiments with uranium.
I have also encountered the stigma of trying to get some and having the general populace thinking you're trying to build an atomic bomb. Apparently
most people don't know what "deleted" means.
It's a real shame people can voice such loud opinions without have any clue what it means and then be taken seriously.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
If someone wants to get some uranium than it's aint so hard these days. On ebay a lot seller will help you to get a small or a larger size of uranium
containing mineral for a relative low price.
Also there are other sellers who offer reagent grade uranium compounds, usually oxides and nitrates, mainly uranyl-nitrate what is a perfect starting
point for experiments with this awesome compound.
And there is the third mentioned way with the vazeline glass, which has a really big problem with it: the glass. Getting out the few percent of U from
that amount of silica requires a lot work and produces a lot toxic waste, so if I would need some U compounds I wouldn't choose that way.
Long ago I wanted to get a small sample of U on my shelf, so I've got a small sample of Uranophane from ebay for a few bucks. It contains more than
50% of U:
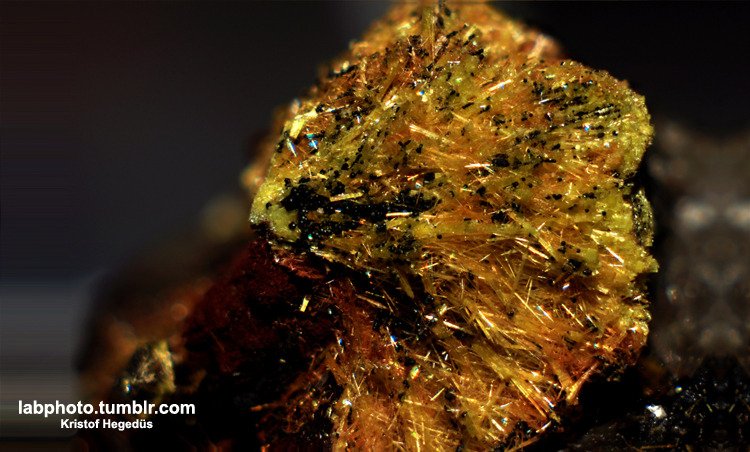
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
GammaFunction
Hazard to Self
 
Posts: 78
Registered: 28-1-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by dontasker  | I've been occasionally looking for similar stuff. ...
I have also encountered the stigma of trying to get some and having the general populace thinking you're trying to build an atomic bomb. Apparently
most people don't know what "deleted" means. |
I can get you as many bottles of deleted uranium as you want. I sell it by the liter, not by the kilo, however. 
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
lots of seller on ebay list some ore as geiger counter test source..the more active the source does not mean more U but other way more active daughter
isotopes (like Radium) so you could probably get a low activity source with a descent amount of Uranium for a relatively small price..
grounded and treated the metal can be extracted by home chemist...I would love to do it (or even go mineral hunting!) but life job and bills keep me
far away from fun as it gets!
there was a thread i think on here about extracting U metal from its ore....
|
|
|
SM2
Hazard to Others
  
Posts: 359
Registered: 8-5-2012
Location: the Irish Springs
Member Is Offline
Mood: Affect
|
|
ruggles mine
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Sorry for the "spam", but here is a recently made pics from my uranophane sample, it looks simply amazing!

Uranophane contains ~55% uranium, so if someone wants to extract some pure uranium, than this could be a good (legal) source.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
That's a great pic- I assume the yellow fibres are the mineral uranophane. What are the black bits that look like beads?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
I'm thinking Uraninite but the bead formation is intriguing. Then again many samples of Pitchblende I have seen are botryoidal instead of octahedral
crystals so I guess not so surprising after all. I do not recall ever seeing it in the form of many tiny perfectly shaped beads like that but I have
never had a sample of Uranophane to study.
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
Zephyr
Hazard to Others
  
Posts: 341
Registered: 30-8-2013
Location: Seattle, WA
Member Is Offline
|
|
I was looking into going mineral hunting for uranium at the Midnite open pit Mine here in washington but the legal implications/restrictions are very
stringent.
Although I don't have any from them, Untied Nuclear offers depleted/undepleted uranium at very affordable prices.
some of their products include:
*3g Uranium metal, price varies on availability but <$40
*5 dram vial of uranium ore $18
*1/4lb chunks of ore, prices vary but are cheap when compared to other sources. ore types include toberite, carnotite, and uranite.
http://unitednuclear.com/index.php?main_page=index&cPath...
This is a procedure which covers the extraction of Uranyl Tricarbonate from uranium ore.
http://www.unitednuclear.com/extract.htm
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Pfft. That's not affordable. Far better to go prospecting, or look on Ebay.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Back then, I was seeing bottle of 4 ounces of uranyl nitrate/acetate from brand like fluka on US ebay, of course they would not sip to Canada... The
bottles where like 150-200$ each.
I never asked for this.
|
|
|
Tdep
National Hazard
   
Posts: 519
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
| Quote: |
I was looking into going mineral hunting for uranium at the Midnite open pit Mine here in washington but the legal implications/restrictions are very
stringent
|
I live in a part of Australia that I can drive in a day to a few uranium mines including Olympic Dam, the world's largest uranium deposit.
I remember looking this up and finding outlandish fines for possession of radioactive substances, something like 10 years jail as well. This act seems pretty against it, not going to lie.I find it hard to believe I can't drive along, find some ore on the side of the road and pick
it up without being arrested. Anyone had any experience with uranium prospecting? 
Note, I do not have the facilities to handle or do anything with radioactive substances. I, at least in the forseeable future, will not ever be doing
anything with uranium but it's on my doorstep and i'm curious.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
I've been waiting forever for them to post Part 2 of that procedure. I want to know how much more processing is involved - I want to be able to
isolate the pure metal from the ore, to add to my element collection.
I've been getting more and more interested in minerals and ores since I got into chemistry, and I think it would be a lot of fun to isolate elements
from rocks. I'm thinking of starting with copper from malachite as an exercise. Sadly, I live on the coast and there aren't any interesting areas for
rock prospecting anywhere nearby that I know of.
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Yer nicer military ammo is made with depleted Uranium. Somewhere in the world, little kids are picking up spent slugs, to sell as scrap.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Copper from malachite is easy - all you need is a furnace.
(I live on the opposite coast - there are plenty of opportunities if you look for them. Or you could go to a rock shop.)
See http://en.wikipedia.org/wiki/Uranyl in the Aqueous Chemistry section for extraction: it says to extract the tricarbonate with kerosene, remove
this layer, and add strong nitric acid (this will form uranyl nitrate complexes which are more soluble in the aqueous layer). Then you could... do
single displacement with aluminum in the aqueous layer?
I have a military surplus store in my town, but I've no idea if it sells ammunition. There is also a gun store, but I've no idea if it sells depleted
uranium bullets.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Aw, never mind. Seems like there might be an ordinance against private citizens using the stuff. Depleted Uranium rounds may have been available
to the public at one time, but I'm not seeing much evidence of such availability now.
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
it seems DU rounds aren't pure Uranium
according to united nuclear
http://unitednuclear.com/index.php?main_page=product_info&am...
"Uranium is used by the Military in projectiles - however it is very impure, being an alloy known as 'Ballistic Uranium'. Ballistic Uranium consists
of Uranium mixed with large quantities of Tungsten, Titanium or Hafnium, which is unacceptable for laboratory use."
|
|
|
dermolotov
Hazard to Others
  
Posts: 114
Registered: 13-12-2014
Location: Toronto, Canada
Member Is Offline
Mood: Free
|
|
There's a bloke on "da intarwebs" that lived beside a depleted Uranium mine and brought out 4 kilogrammes of uranium ore. He washed it with copious
amounts of acids and left it there to digest.
After a few purifying steps, he managed to get a reasonably pure yellowcake.
Here he is:
https://carlwillis.wordpress.com/2008/02/20/uranium-chemistr...
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
As far as I know, the Army has always used U3/4Ti alloy, which is DU with 3/4% Ti. The Navy (in their Phalanx System) uses a 2% Mo alloy. The uranium
should otherwise be highly pure (reduction from UF6 usually produces uranium of 99%+ purity)
DU pentrators are highly engineered, and the alloy performance is critical, so excellent quality control should be in place.
There is no reason that I know of that tungsten or hafnium would be present - I don't recall ever seeing a report on uranium ballistics that mention
them.
|
|
|