AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I can see the color spectrum?
This post is about color perception at different frequencies in the spectrum.
I have a something I have been pondering. I have been comparing energy-saving LED replacement bulbs to regular incandescent bulbs, and they are just
not the same. I prefer incandescent light. I know that the frequency spectrums are different, so it should be no surprise that the two render colors
differently, but what puzzles me is the overall color of the light. Theoretically, the two should look very similar illuminating a white surface, yet
I can see a significant difference.
I have a Philips "2700K" correlated color temperature LED lamp. The light it gives off seems yellowish-orange, with a purple tint. The strange thing
is, it is almost as if I can "see" the color frequencies this LED is deficient in. Although the light is a orangish-yellow, it still does not
feel warm. It is more like a fake fluorescent yellow color. It is like I can sense the deficiency in red frequency light.
I also have a "3000K" LED replacement bulb that is whiter in color. But it has a pinkish tint to it, and somehow it does not really seem brilliant
white in color. When I think about it, the light color seems deficient in the color green.
What I do not understand is that supposedly the eye has only 3 color receptors: red, green, and blue. How is it that I can sense the deficiency of
deep red frequency light? If the light is orangish yellow, should it not be activating the red receptors in my eye? Or if it activated the green
receptors more, then why does LED light appear to have a purplish tint?
Also, both of these LEDs use the same technology, a gallium nitride blue LED chip with Nd:YAG phosphor, the only difference is how much phosphor the
blue light is passing through. The ratio of "red" to "green" should remain constant in these LED lamps, whatever the "correlated color temperature".
When a typical white LED is increased in "color temperature", it essentially just means the ratio of blue frequency light in the spectrum is
increased, whereas when the temperature of an incandescent filament is raised, the whole color frequency spectrum shifts towards the blue.
I have also compared the "3000K" LED to a 2960K halogen bulb, and the color contrast becomes very apparent. The halogen bulb seems to have a much
greener tinted color of light in comparison, and is also that brilliant white color the LED is lacking.
The "pinkish" or "purple" color of the LED light must no doubt be caused by the higher ratio of blue frequency in LED light. The color
magenta would probably be more accurate. The blue (465nm) frequency peak in LED light is also of a shorter wavelength than most of the
frequency range of blue light from incandescent sources, so it is more towards the indigo/violet end of the spectrum. To some extent, this can be
explained by the fact that the black body curve in normal incandescent light is centered on yellow, whereas the hoter temperature in halogen light
centers the top of the curve into the green.
The concept of color coordinates means that any color perceived by the human eye is basically just a ratio of red, green, and blue. It should not be
surprising that LED light has a somewhat different color coordinate than incandescent light, even for the same correlated color temperature.
How can it be that LED light neither feels warm, nor seems to be a brilliant white? Why is it that I can perceive that LED light is deficient in red
and green-blue frequencies? The 3 color receptor model of the eye fails to explain this. Why do LED Christmas lights seem to have a
fake fluorescent greenish yellow feel?
http://www.wronkiewicz.net/mars_colors/human_cone_action_spe...
I also read that some people have different copies of the red receptor gene, so the response peaks at slightly different frequency between different
people.
http://en.wikipedia.org/wiki/Tetrachromacy
http://www.dailymail.co.uk/sciencetech/article-2166357/How-c...
http://www.post-gazette.com/stories/news/health/some-women-m...
[Edited on 4-2-2013 by AndersHoveland]
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
I think it is merely the ability to discriminate and process across the visible spectrum built into us. Tailored to see 'natural color' in the light
of our specific type star. Since all of these modern light sources produce light with peak intensities higher in various specific energy levels and
weaker at other frequencies, our ability to finely discriminate comes into play. Light produced by hot filaments or hot plasma (the sun) is going to
be much more uniform in intensity across broader frequency ranges. Even though the sun has a high intensity in the yellow our eyes have a peak
response in the green. I believe it is a mechanism to allow us to compensate for the spectral variations so that we perceive a natural color spectrum.
We are used to seeing things in a more random flux of light. In effect our extremely fine ability to discriminate makes power peaks in a few narrow
windows of frequency coming from these new light sources cause us to perceive a very un-natural lighting. I may have worded this poorly but I think I
got my thought across.
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
Twospoons
International Hazard
    
Posts: 1302
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
Do you notice this when just when looking at white surfaces? It may be that the "white" you are looking at also has spectral peaks and valleys in its
reflectivity, and so would look different under peaky LED light, compared to incandescent. How do you get on with fluoro tubes? Some of those are
horribly peaky, especially the cheap ones.
Don't forget that some "whites" include a fluorescent blue component to balance out a yellow substrate (white cloth for example), which would probably
be overly responsive to the LED blue peak.
Out of interest, whats the CRI of the LEDs?
Nicest lights i've ever had were specially filtered 'daylight' halogen spots I bought for my wife's art table. You'd swear you were looking at
sunlight from a hole in the roof.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 859
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
This might also just be confirmation bias. It would be very interesting to set up a blind experiment where you'd have to identify the missing colors.
This just in: 95,5 % of the world population lives outside the USA
You should really listen to ABBAPlease drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Fluorescent is the worst of all. The warm white ones have either an unpleasant reddish pink tint, or a sickly yellow-green tint. It makes colored
objects appear greyish and off, and also strains my eyes.
The frequency spectrum for fluorescent CFLs is just awful. It is not continuous at all, which means colors often look wierd under fluorescent light:
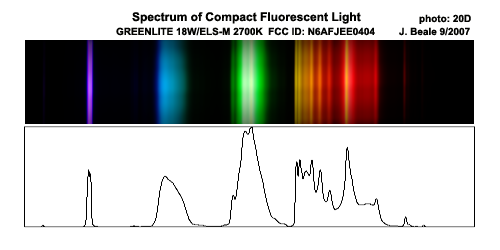
This graph does not show the UV, some of which leaks through the glass and results in yellowing of the plastic base of the CFL over time. The
frequency of UV given off by fluorescent tubes is mostly at 253.7 nm, some at 365.4 (UVA), and a tiny ammount of 184.5 that manages to get through the
thin glass.
Here's the spectrum from an incandescent light bulb for comparison:

As you can see, light produced by incandescence is full smooth spectrum, without any sharp spikes.
I do realise that there exist a small number of fluorescent tube products that actually closely match the spectrum of sunlight. True Lite
brand is one of them. Unfortunately most of the other fluorescent products that claim to be like "natural daylight" grossly exaggerate.
Fluorescent is not really an option for me. Those spiral CFL bulbs make my skin feel sore, probably because of the UV they leak out. I can begin to
feel it after 10-15 minutes of being under one. It is not really a painful feeling at first, it feels more like my skin is being roughed up. It
continues to feel irritated for up to 24 hours afterwards. If I sit near a lamp with one of these CFLs behind the lampshade, the side of my face
facing the lamp feels sore. Some individuals apparently are more sensitive to this than others. The strange thing is I do not seem to have as much
problem with being out in the sun. It is the same thing with bare exposed fluorescent tubes. I can tolerate fluorescent lighting in offices with
acrylic coverings, but my skin still feels irritated if I am in these settings for the whole day. Obviously this leads to a significant ammount of
inconvenience for me, especially with many stores now changing out their recessed lighting fixtures with exposed CFLs.
I think the issue of UV emissions from CFL bulbs has not been adequately addressed. I do not want to start a controversy here, but I will just say
that there do exist some political/social reasons why the government and media might be choosing to minimize or ignore these problems. At least this
Australian health site suggests some ways to minimize exposure from CFLs in ones home:
http://www.climatechange.gov.au/what-you-need-to-know/lighti...
Quote: Originally posted by Twospoons  |
Nicest lights i've ever had were specially filtered 'daylight' halogen spots I bought for my wife's art table. You'd swear you were looking at
sunlight from a hole in the roof. |
Yes, halogen lamps are generally regarded as having the best quality of light (besides real sunlight of course). Halogen is just a form of
incandescent light, but typically with the filament glowing at a higher temperature. Typically 3000 °K is the hotest the filament can be designed for
without unacceptably sacrificing lifetime. At much lower voltages the filament can be thicker, allowing it to operate at 3500 °K and still have an
acceptable lifetime.
A brand called SoLux makes directional halogen lights that also closely matches the spectrum of sunlight. They use some type of coating that
reflects the shorter wavelengths and allows the longer wavelengths in the spectrum to pass through, where they are absorbed by a painted black
coating. I suspect that the coating utilizes Rayleigh scattering, similar to the phenomena that causes the sky to be blue. While sunlight is typically
5000 K, due to greater scattering of the shorter wavelengths, the light spectrum in the shade or for an overcast sky can basically match a black body
curve at 9000 K.
The CRI of normal Nd:YAG white phoshor LED lamps is directly tied to the correlated color temperature rating. The LED chip is just emitting a narrow
blue frequency peak. It is the yellow phosphor that gives off a broader spectrum of frequencies. A 3000K CCT LED will have a CRI rating of 80. Some
"enhanced spectrum" LED lamps use separate red frequency chips to boost the CRI rating to 92. These are more expensive.
The Philips L-prize is one example of this. Unfortunately, this product is using 635nm frequency red chips for slightly higher efficiency instead of
common 660nm red chips. While the human eye is more sensitive to 635nm, this still overlaps with the broad spectrum hump from the Nd:YAG phosphor and
does not do a great job of rendering deep red frequency colors. Using 660nm would give better color rendering (94 CRI).
Of course, red frequency spectrum enhancement will only make the overall light color problem worse. LEDs still have some decificiency of green-blue
frequency light, at least relative to the deep blue spike. To take the magenta tint away and create a more brilliant overall white color, green light
needs to be added. 505nm blue-green LED chips are relatively common (used in many traffic lights). A 505nm spike in the LED spectrum closely overlaps
with the yellow-green side of the ND:YAG phosphor hump. Adding a very small ammount of 505nm light would initially slightly increase CRI, but
thereafter lower it. Improving the overall color ratio of the such a combined LED lamp would come at the cost of spectrum smoothness and CRI. A 505nm
spike would still barely fill in the green-blue frequency depression. Turquoise (495nm) LED chips exist, and would be ideal to help fill in the
frequency deficiency in this range, but unfortunately they are uncommon right now and more expensive.
I do not believe CCT or CRI ratings are adequate to really describe the quality of light. The frequency spectrum of a non-black body light source is
too complex to be expressed with simple ratings like this. "Color temperature" does not necessarily indicate what overall color the light source
actually is, and there have been several criticisms that the standard CRI rating does not correlate well to actual color rendering ability.
[Edited on 4-2-2013 by AndersHoveland]
|
|
|
Twospoons
International Hazard
    
Posts: 1302
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
Yes, that's the one! Truly wonderful lights.
Quote: Originally posted by AndersHoveland  |
"Color temperature" does not necessarily indicate what overall color the light source actually is, and there have been several criticisms that the
standard CRI rating does not correlate well to actual color rendering ability.
[Edited on 4-2-2013 by AndersHoveland] |
I've heard this too. Its probably because CRI wasn't developed with discontinuous spectra in mind.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
The reason I am probably finding LED light to look somewhat unnatural is likely the simple reason that current LED technology still has a long way to
go:
http://www.open-photonics.com/featured-technologies/high-cri...
http://www.verbatimlighting.com/article/VxRGB-violet-chip-te...
Virtually all of the currently commercially available LED products just use a blue LED chip with an orangish-yellowish phosphor (it's not
really white light) so it should not be surprising that none of these LED products really compare to the warm pleasing glow of an
incandescent bulb.
This image represents the spectrum of any regular LED retrofit bulb you will find in a store:

[Edited on 30-4-2013 by AndersHoveland]
|
|
|
|