Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Colors of hydrogen balloon flames during lectures
I didn't want to name this thread "hydrogen flame color" because it deals with messy environments and not with the science itself.
Please watch this, first.
<iframe sandbox width="560" height="315" src="http://www.youtube.com/embed/qOTgeeTB_kA" frameborder="0" allowfullscreen></iframe>
Periodicvideos channel is great stuff, but they occasionally fail in making scientifically correct videos.
I was immediately struck with the bad science in this episode.
It's like they failed to realize a couple of things like the fact their pressurized stoichiometric mixture in the balloon detonated with the same
color as the pressurized hydrogen deflagrated, but it was whiter because the power (energy over time) of the reaction was much greater. The camera was
blinded, but yellow is still there. They just ignored it.
It's like they're calling "white" a color. How scientific is that? Not at all.
Their explanation is something like: "Pure hydrogen glows yellow-orange when ignited, and stoichiometric mixture is glowing white because there's more
energy involved".
Absolutely bonkers, kiddie-level "leave me alone, the sky is blue because god made it that way" explanation.
AFAIK, hydrogen diffusion flame in an oxygen rich environment is bluish, though very weak and pale.
The more you premix it with oxygen, the more UV is given off.
(Of course, the same thing would happen if we lived in a hydrogen atmosphere and we used oxygen in our burners. Let's keep
this discussion in the field of science, not dumbed down engineering where fuel and oxidizer are treated as something fundamentally special.)
They've planted an erroneous explanation in lots of brains and now you've got people talking about hydrogen glowing like a black body, and people
talking about stars being yellow. A complete cacophony of bad science. 
The color of the flame comes from the excited, free molecules or atoms, essentially a low temperature plasma. Neither hydrogen, nor oxygen, nor water
vapor nor any possible intermediate wild particle can give off light of such colors.
Every time I see someone doing this experiment with ballons, the color is more or less different and it goes from yellow to orange-red. It means it
must be because of contaminants.
Balloons have that dust inside. I don't know what that is, and whether it's always the same substance, but cornstarch is often mentioned.
Snapping balloon is a source of cornstarch aerosol which, when ignited, gives off a full spectrum of incadescent carbon. A simple peek through a DIY
grating spectrometer would be very helpful.
Also, the gases used in quick lectures are never very pure. Sodium compounds in a form of aerosol are often a source of irritating contamination.
Basically, they've made a fundamental mistake. You can't use basic chemical theories to explain higher ordered phenomenons. They're simply too narrow
to grasp them.
That's why we can't use the theories that explain basic particle kinetics to explain the stock market.
Granted, flames are much less complex systems than the stock markets, but they're above the basic "2 H2 + O2 -> 2 H2O".
What they need to do is to get in touch with someone studying combustion and flames on a scientific level. A pyrologist, if I'm correct.
Comments?
[Edited on 13-1-2013 by Endimion17]
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
I quite agree. My teacher deflagrated a small amount of hydrogen a few weeks ago, which seemed to burn with an intense, pure-red flame.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Heuteufel
Harmless

Posts: 25
Registered: 5-11-2011
Member Is Offline
Mood: No Mood
|
|
An easy way to avoid the whole problem: Flame colours in burning hydrogen 
|
|
|
per.y.ohlin
Harmless

Posts: 27
Registered: 14-11-2009
Member Is Offline
Mood: No Mood
|
|
The orange is probably from a temporary lack of oxygen, or sometimes some dust. I'm fairly certain that the blue is always there, just it is very
faint in comparison to daylight.
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Can we talk about hydrogen, please? Methanol is a compound, a carbon compound.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Yes, a hydrogen-rich carbon compound . . .
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Tidbits
"According to Dr. Robert Pitz from Vanderbilt University, "Pure hydrogen (with no sodium) -- air flames will glow red in a dark room due to the water
vapor emission lines." Both Dr. Joseph Wehrmeyer working in support of the Air Force and Richard Eskridge from NASA concur, noting that water vapor
generates an orange-red-infrared continuum in such flames. However, all of these individuals also noted that there is a strong orange coloration in
such flames due to sodium contamination within the hydrogen. The sodium is present as sodium hydride within the liquid hydrogen which decomposes at
high temperatures to generate the vibrant color. The sodium contamination is a byproduct of how large, industrial quantities of hydrogen are made
for uses such as, for example, flying the Space Shuttle. Dr. Christopher Dobbin of NASA noted that in the 1990 timeframe he was engaged in an
analysis of the flame plumes ejecting from the Space Shuttle Main Engines (SSME). He said, "The (time) average sodium concentration we measured in
the SSME exit plane was 0.091 parts per billion." That doesn’t sound like much, and it's not enough to impact engine performance or operation, but
it's still enough to measure based upon spectral analysis of the plume. Another possible contaminant, according to Richard Eskridge, is potassium and
that can further contribute red emissions."
http://blogs.nasa.gov/cm/blog/J2X/posts/post_1332790109254.h...
http://blogs.nasa.gov/cm/blog/J2X/posts/post_1329851305074.h...
http://en.wikipedia.org/wiki/File:Shuttle_Main_Engine_Test_F...
Page 6
"The hotter oxyhydrogen flames also emit the Schumann-Runge O2 bands between 250 nm and 400 nm, and a weak continuum in the blue and ultraviolet
regions of the spectrum. This continuum, which in the absence of impurities practically does not exist in the spectrum of air-hydrogen flames ..."
http://www.tempe.mi.cnr.it/zizak/tutorial/cairol06-flame-emi...
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Still, a carbon compound, therefore it's interfering. Burning methanol gives different intermediate excited molecules. Methane is also a hydrogen rich
compound, but its diffusion flame produces full spectrum incadescence.
Hydrogen is a very special element. Its atoms are so simple and tiny. Only one electron in a tight cloud.
@Morgan: Thank you so much for the links. There's the spectrum, too. Excellent. 
So I was correct. Using pure reactants, no yellow and orange color should be seen. If the total energy, reactant flux and density are high enough, the
water molecules give of enough near infrared to be picked up by a digital camera which registers NIR. Very dull read appears in darkened room to the
human eye, too.
The balloon experiments in the video are very different from these torches. Absolutely no yellow-orange light should be seen.
I'll try to conduct a series of experiments using pure and contaminated hydrogen in the balloons. From my experience so far, really pure hydrogen
flame is really difficult to see.
I think it's obvious there's a contamination from the balloon itself at this point. If it's incadescent light, it's cornstarch dust.
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
If the H2 and O2 are pure you could look for some of these players. Calcium nitrate kind of jumps out at you. Maybe the Mg silicate talc would emit
some white.
"A commonly used coagulant solution is a mixture of water, a calcium-based salt, soap, and talc powder. The salt is the actual coagulant; the soap
helps the latex spread in an even film, and the talc helps ease the removal of the rubber from the forms in a later step."
http://www.madehow.com/Volume-2/Balloon.html
"The primary ingredient of the coagulant is Calcium NItrate"
"Insoluble CaCO3 calcium carbonate works well for this purpose and is easy to find. The finer the powder the better! I have also heard that talc works
for this purpose but I have no experience with it in coagulant."
http://balloonseller.com/makecoagulant.htm
Maybe the cleaning solution ...
http://www.youtube.com/watch?v=y8oWkx1PhUY
[Edited on 16-1-2013 by Morgan]
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
More hydrogen than hydrogen.
"With the chemical structure CH3OH, methanol is the simplest alcohol, with the lowest carbon content and highest hydrogen content of any liquid fuel."
http://www.methanol.org/Energy/Resources/Alternative-Fuel/Me...
Maybe because of the nearly invisible flame one could use this quality to test for some of the trace contaminants in a balloon, if indeed that is what
is creating the pronounced colors in the video and not the hydrogen. I have a large tank of O2 but methanol really goes off with a bang in pure O2 and
I don't have a high speed/slow motion camera. I could capture a flash however and a clear/white balloon would probably be best if it fires before the
balloon breaks. Maybe if you rinsed out a balloon and then compared the results to an ordinary powdery one as well. Or simply see what color a little
methanol burns after a swirl inside a balloon, that should pick up something. But an explosion would be more illustrative. I know all this introduces
more variables and it wouldn't be the same experiment as hydrogen, but you could glean some things from methanol as ideas occur to you.
Another tenuous variable might be the type of balloon or what part of China it was made in for example. ha
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Yes, methanol has more hydrogen atoms than a hydrogen molecule, but it contains other stuff as well, and that's the only important thing. It can't be
used for determining what's the case with hydrogen.
Hydrogen and oxygen can't give lots of different intermediate excited particles, but methanol might. I'd say there's plenty of choice.
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
The test might be the old oxy-hydrogen welding flame, which is reported as being colorless.
Reminds me of an old chem discussion regarding a lithium flame test. The first question to be answered is "Why is the Bunsen Burner flame, Blue?"
Answer at the time= Because of the Carbon Dioxide being formed. The second question is "Why is the top of the Bunsen Burner flame, Orange?" Answer
at the time= Glowing soot particles from incompletely combusted hydrocarbon gas.
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Yet another slant, a liter of liquid hydrogen weighs 71 grams whereas a liter of methanol contains 99 grams of hydrogen.
http://en.wikipedia.org/wiki/Methanol_economy
True, methanol wouldn't be the same as hydrogen. But you might pick up some colors from the balloon if you think the balloon is imparting red, orange,
or yellow. A rough comparison could be made with a polyethylene baggie balloon.
Next there's the hydrogen contamination possiblity, the fuel/air ratio with temperature variables, density of gases according to the NASA article,
camera sensitivities, and most likely other aspects and correlations not yet revealed.
In this clip there is this last one shot outdoors where the red is quite pronounced. I didn't see any yellow. It seems to be a weaker explosion.
http://www.youtube.com/watch?v=qOTgeeTB_kA#t=37s
Just some aurora tidbit about oxygen.
"The red line of oxygen (630 nm) is dominant at high altitudes, but fades below 150 km, as the excited oxygen atoms in the 1D-state are de-excited by
collisions with nitrogen molecules much faster than by radiation."
(Bottom of page)
http://www.itp.uni-hannover.de/~zawischa/ITP/atoms.html
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
If hydrogen burns with a colorless or a faint reddish flame, then the effect must be weak. I have made hydrogen by adding a piece of magnesium to
dilute hydrochloric acid in a full test tube and letting the gas push out the dilute acid. Then with a syringe I sucked the hydrogen out of the test
tube, while keeping it inverted under water. Then I attached a pasteur pipette to the syringe and slowly pressed the gas into the air. When the gas is
ignited then it burns with a nice small orange flame. Not orange like a flame of butane with insufficient orange, it is clear that the flame is due to
glowing gas.
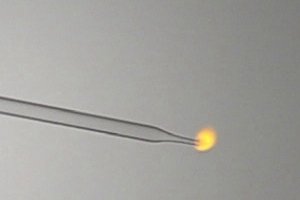
I did a similar experiment with methylnitrite, which burns with a weakly colored grey flame. This also was made in a similar way in an environment
without sodium. Now, solid KNO2 was added to a dilute solution of methanol/HCl in water. When KNO2 is added, it starts bubbling and nearly pure CH3NO2
(not to be confused with nitromethane, this is an isomer!) appears. The gas presses the solution out of the test tube again and fills the test tube.
When this gas is ignited in the same way as hydrogen, then a pale grey flame can be observed.
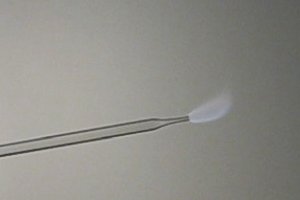
Now, I ask myself, if the orange color of burning hydrogen is due to sodium contamination, then why is the color of the burning hydrogen orange and
the color of the burning methylnitrite is not? Both were produced in very similar ways. I used the same HCl for making hydrogen and methylnitrite.
I a counter experiment I also made methyl nitrite from sodium nitrite. If I burn this gas, then the gas burns with an orange flame.
Should the conclusion be that the color of a burning hydrogen flame is MUCH more sensitive to sodium contamination than the color of a burning
methylnitrite flame?
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
woelen, I've commented your YT video on this experiment. This is sodium contamination. The glass at the tip is melting. It's a very common situation.
Methylnitrite flame is probably not hot enough to cause the evaporation of the sodium from the pipette.
Morgan, the outside illumination is far greater than indoors. It's a digital camera, a commercial model.
True scientific experiment would involve setting everything to "manual", but most digital cameras don't give that opportunity to the user. I've seen
many crooked colors of same phenomena in digital video, all because some things are set to "automatic".
The aurora thing... I think it's irrelevant to our case. The conditions are quite different. This is burning. Auroras are plasma. The energy level
transitions are quite different.
[Edited on 17-1-2013 by Endimion17]
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I found this when I was trying to find more info on oxygen, just entertaining the possibility that oxygen might somehow be contributing a fraction of
the color in the balloon bursts.
"A red luminescence can be obtained due to the formation of singlet oxygen. Singlet oxygen causes the red light that can be seen during polar lights
(aurora borealis). All in all the experiment shows a chemical "artificial" polar light."
http://www.youtube.com/watch?v=coX0sHTj21I
http://www.youtube.com/watch?v=vMi8kKXgshk
Chemical oxygen iodine laser
"The aqueous peroxide solution undergoes chemical reaction with chlorine, producing heat, potassium chloride, and oxygen in excited state, singlet
delta oxygen. Spontaneous transition of excited oxygen to the triplet sigma ground state is forbidden giving the excited oxygen a spontaneous lifetime
of about 45 minutes. This allows the singlet delta oxygen to transfer its energy to the iodine molecules injected to the gas stream; they are nearly
resonant with the singlet oxygen, so the energy transfer during the collision of the particles is rapid."
http://en.wikipedia.org/wiki/Chemical_oxygen_iodine_laser
[Edited on 17-1-2013 by Morgan]
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Just some attempts at understanding ...
Ozone is produced by ultra violet light splitting O2. Then O + O2 = O3. When H2/O2 balloons detonate, there is a burst of UV rays. I wonder if there
could be some sort of cascade, a partial photolysis/synthesis, before hydrogen reacts fully, maybe moreso with slower times in a hydrogen/air mixture?
I recall the professor saying "Paul" thought in a hydrogen rich balloon the "heat" from the first part of the flame ..." . I think he was saying the
Balmer lines of hydrogen are being excited, there's a short scene of his email letter.
But maybe oxygen could be responsible in part for the red, dropping in energy. I'm not saying Paul is right or wrong or even if I understood the
explanation, I'm just feeling around. In any case it would seem if a UV burst could split O2, then that would accelerate the reaction with hydrogen,
possibly leaving an O to be hit with UV. Or maybe an H. Here's that clip.
http://www.youtube.com/watch?v=qOTgeeTB_kA#t=1m11s
Tidbit
"In a similar way, ozone is destroyed by solar radiation. Ultraviolet radiation hits ozone and breaks it back down into molecular oxygen (O2) and
atomic oxygen (O). The oxygen atom O then reacts with another ozone molecule to form two oxygen molecules."
http://www.atmosphere.mpg.de/enid/1z1.html
I wonder if high temperatures from inside the H2/O2 balloon/cloud along with a UV energy burst and expansion/rarefaction of the gases would
compensate/overcome other factors, thus allowing some briefly remaining oxygen to produce red light as it does at high altitudes where fewer energy
draining collisions allow the effect?
"Most auroral light is from excited oxygen atoms. Above 100 km the atmosphere is mainly oxygen atoms and nitrogen molecules, the molecular oxygen is
dissociated into atoms by solar extreme ultraviolet light."
http://www.atoptics.co.uk/highsky/auror3.htm
[Edited on 18-1-2013 by Morgan]
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
The Photochemical Hydrogen-Oxygen Reaction
"The products of the reaction produced by the absorption of ultraviolet light by hydrogen-oxygen mixtures are ozone, hydrogen peroxide, and water."
http://pubs.acs.org/doi/abs/10.1021/ja01308a014
Examination of the Gases produced by Burning Hydrogen in Air
http://books.google.com/books?id=UhlLAAAAYAAJ&pg=PA67&am...
[Edited on 18-1-2013 by Morgan]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Every few years my father would bring home mixed metal salts to throw on our wood fires for the color experience.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Three Balloons
http://www.youtube.com/watch?v=a6qGIMqDKwA&NR=1
|
|
|