morry-cule
Harmless

Posts: 21
Registered: 16-12-2012
Member Is Offline
Mood: No Mood
|
|
Synthesis of 4-phenyl-L-proline
Hi Sciencemadnessers!
I'm new to the forum, used the search engine, came up empty handed.
I've read through Wiley's 10th Ed. O. Chemistry but still I'm at a loss as to what I'd need to synth 4-phenyl-L-proline.
Any help would be appreciated. This isn't for homework or class or anything. My own personal project.
Attachment: 4-phenylproline.sk2 (1kB)
This file has been downloaded 724 times
|
|
|
VitaminX
Harmless

Posts: 17
Registered: 27-11-2012
Member Is Offline
Mood: Moody
|
|
Well, are you looking to synthesize cis- or trans- 4-phenyl-L-proline?
For cis, you should look into:
Tamaki, Makoto; Han, Guoxia; Hruby, Victor J.
Journal of Organic Chemistry, 2001 , vol. 66, # 10 p. 3593 - 3596
Very good and detailed information there.
Trans:
Krapcho, John; Turk, Chester; Cushman, David W.; Powell, James R.; DeForrest, Jack M.; et al.
Journal of Medicinal Chemistry, 1988 , vol. 31, # 6 p. 1148 - 1160
Both start off with 4-hydroxy-L-proline
|
|
|
morry-cule
Harmless

Posts: 21
Registered: 16-12-2012
Member Is Offline
Mood: No Mood
|
|
Well I imagine that cis-4-phenyl-L-proline would be the more active isomer. I'm basing this on phenylpiracetam which is a racemate, its cis equivalent
image implied as the active isomer.
Problem is I plan to attach a peptide bond (glycine ethyl ester) to its C-terminus to create a kind of noopept-meets-phenylpiracetam compound and I
don't exactly know whether a cis equivalent of 4-phenyl-2-oxo-pyrrolidine will work as I intend.
How do I access your above links? I was told I didn't have authorization and to pay $35.00 for 48 hours. Surely your above info was intended as to be
accessed free of charge. I'm new to JOC searches and such, so please pardon my ignorance. I'm still a sapling in this forest of chemistry... 
|
|
|
VitaminX
Harmless

Posts: 17
Registered: 27-11-2012
Member Is Offline
Mood: Moody
|
|
I don't see where you get the 4-phenyl since neither Noopept nor Piracetam have a 4-phenyl moeity.
Also be careful with just jamming random molecules that you like together and thinking something useful will come out of it.
Most of the times it doesn't work that way.
Anyway to respond to your question:
http://www.file-upload.net/download-6944436/prolin.rar.html
Here you go with the papers.
|
|
|
morry-cule
Harmless

Posts: 21
Registered: 16-12-2012
Member Is Offline
Mood: No Mood
|
|
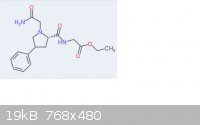
Attached is the structure of what I'm talking about.
Phenylpiracetam (not piracetam) has the 4-phenyl moeity.
I understand what you mean about putting random "frankenmolecules" together. That would be fairly unproductive and dangerous. In this case though the
nootropic Gly-Pro sequence has limited abilities crossing the BBB without support of a polar moeity somewhere. With positive allosteric AMPA
modulators a phenyl group is used in various positions away from the pyrrolidine nucleus.
See aniracetam, noopept, sunifiram, phenylpiracetam, and nefiracetam. All put the phenyl group in different positions away from their respective
pyrrolidine group yet all act as (+)AMPA mods. I'm merely using phenylpiracetam's positioning of its phenyl group so the whole compound can penetrate
the BBB, otherwise it wouldn't.
I hope that makes sense. Thank you for that file, I'll check it out!
|
|
|
Nicodem
|
Thread Moved
19-12-2012 at 08:07 |