| Pages:
1
2 |
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Recovery of Cesium salts from Pollucite ore.
Cesium salts are expensive and that is the limiting factor to my involvement with this alkali metal. But I found a simple method to extract
relatively high purity cesium salt from its chief ore pollucite. If only I had a source for pollucite, might have to go rock hunting, but I digress.
Procedure from the series entitled "Inorganic Syntheses":
| Quote: |
Cesium Alum Method
Submitted By Robert West and Robert P. Anderson
Checked by Lewis I. Krimen and Therald Moeller
Pollucite is broken into pea sized lumps with a hammer and then ground in a ball mill until fine enough to pass though a 120-mesh sieve. One hundred
grams of screened pollucite is mixed with 400 ml of 50% sulfuric acid (7.1 M) in a 1-L round-bottomed flask and refluxed gently for 30 hours. The
mixture is diluted with 250 ml of water, heated to boiling, and filtered with suction, using a large, coarse, sintered-glass funnel. The silica
residue is washed well with hot water.
The hot solution is cooled to 0C, and the well-defined octahedra of cesium alum are collected on a sintered-glass funnel and washed with cold water.
A small second crop of crystals is obtained when the filtrate is concentrated to about 450 ml and cooled. The yield is about 110 g., or nearly 90%
(for Varutarask pollucite).
Properties
Cesium alum crystallizes in colorless octahedra which melt at 117 C. This compound is more insoluble then the other alkali-metal alums and is
remarkable for its high temperature coefficient of solubility. At 100C, 12 is soluble in 100 g of water; at 0C, only .19 g is soluble. Because of
this property, the cesium compound is readily prepared in a high state of purity by recyrstalizing it from water. |
An interesting and straight foreward procedure.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I also like the idea of extracting metals from ores - it is just so fundamental.
If you don't want to invest in a ball mill you can buy an 8 oz capacity iron mortar & pestle for <$30 at http://www.minerox.com/ I have one and it did a nice job of pulverizing a 1/2" piece of gravel from my driveway.
[Edited on 2-8-2004 by Magpie]
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Re: Caesium
Pollucite, the principal (silicate) ore of Cs mentioned, is itself of fairly rare occurrence.
How about looking at the possibility of extracting Cs from sea-water? I think that, like Li and Rb, it occurs in sea-water at about 1 ppm levels. Of
course, as an alkali metal, its occurrence there is greatly surpassed by the Na and K. Like Li, it could conceivably be extracted, along with Rb, from
sea-water with some sort of ion-exchange resin, from which, when packed in a column, the much larger fractions containing all the Na and K could be
discarded.
Although the physiological action of Cs and Rb in plants is similar to that of K, which is essential to and concentrated by plants, there may be some
plant species which selectively absorb Cs and Rb in preference to K.
John W.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
If this table http://www.seafriends.org.nz/oceano/seawater.htm
is right, then the concentration is 0.0003 ppm. That means that for each gram of Cs you need about 3000 tons of seawater.
Have you got a big bucket?
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Cs and Rb in seawater
3 grams of Cs in 3000 tonnes may not sound much, if you have read it correctly, but with sufficiently large and efficient pumps, and sufficiently
efficient ion exchange resins (if they could be found) to separate Cs and Rb from the Na and K, it should be feasible. I have heard of plans to
extract elements like Au and Th, found in even lesser concentration, from sea-water, and of ion-exchange separation already being used to obtain Li
from sea-water.
John W.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
So are there any operations where you're already processing seawater on vast scales and could get additional minerals as an almost-freebie? The
closest I know of is the extraction of bromine from seawater, but bromine is far more abundant than cesium. I've read of pilot-scale Japanese
efforts to extract lithium, uranium, and vanadium from seawater, various schemes ranging from really-kooky to conceivable for gold from seawater, and
proposals for other metals, but nothing that's reached full-scale production.
PGP Key and corresponding e-mail address
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Lithium is about 7 times as expensive as cesium and about 500 times more common in sea water.
There is a real demand for lithium for industrial purposes and for the nuclear and pharmaceutical business. There is also a real demand for bromine,
perhaps less so than when it was added to leaded petrol, but real.
The demand for cesium is a few chemists in research labs and a few physicists with clocks. If you were able to double the world's supply of
cesium you would pretty much halve the price.
I don't think the ecconomics don't make sense.
Also, if I shift that 3000 tonnes of water I can hope to extract a gram of the stuff., worth about £0.82 which would buy me about 40 KWh of energy
(based on my gas bill). I'm not sure that 40Kw would process 3000 tonnes in an hour, its only enough to lift the water about 15 feet clear of
sealevel.
[Edited on 14-8-2004 by unionised]
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
Lithium is more expensive than cesium?! Either you've found a great bargain-basement cesium compounds dealer or you're paying too much for
lithium compounds!
For example, Aldrich wants $35.10 for 500 g of 99% lithium carbonate and $99.50 for 250 g of 99% cesium carbonate. The price differential is much
smaller with highly-purified materials, presumably because the price of purification is large compared to the price of raw materials.
You can buy technical grade lithium carbonate (for pottery) for less than $16/kg. I've never found any cesium compound of any grade for sale near
that price.
PGP Key and corresponding e-mail address
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
One biochemistry web site sold cesium chloride for the unbelievable price of $175 a kilogram, that for me was pretty cheap.
BTW, I read recently that lithium is one of only two metals that has actually increased in price (when leveled with the consumers price index) over
the last twenty or so years, probably due to the difficulty of processing ore and the highly increasing demand for it.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
"Lithium is more expensive than cesium?! Either you've found a great bargain-basement cesium compounds dealer or you're paying too much
for lithium compounds! "
Or I goofed, and got them the wrong way round.
The point is that cesium is rare and almost useless so it's expensive.
The concentration in the earth's crust is estimated as 27 ppm so you would do better picking up a random rock rather than the same weight of
seawater. (though, I realise the rock would be harder to process).
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
Steps for mining oceanic materials:
1. Create nuclear reactor and steadily improve it until tremendous power output.
2. Buy a few tons of freshly evaporated sea salt, seaweed, and such.
3. Perform a massive electrolysis!
4. Separate the halogens and similar materials and sift through the metal.
5. Sell the metallic parts or save some, and keep the tanks of precious fluorine, chlorine, bromine... and I dont really think you can put iodine in a
tank...
6. Use money to uprade reactor and buy raw sea materials. (Repeat)
7. Repeat until you have all the metals and halogens and chemicals and money you need.
------------------------------------
Yes... I have gone mad...

F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
rift valley
Hazard to Others
  
Posts: 103
Registered: 24-7-2004
Location: NH
Member Is Offline
Mood: semiconducting
|
|
Hmmm. Back in the late 1800's and early 1900's they were various pollucite ore mines in nearby Maine. In the last weeks of summer maybe
I'll take a trip over there and see what I can find.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
According to Römmp, seawater contains about 1 microgram of Cs per liter.
When Bunsen and Kirchhoff (sic) extracted it from Bad Dürkheimer (Germany, Eiffel) mineralwater they needed about 42000L to produce a few grams IIRC.
And that water is supposed to be enriched with it because of vulcanic activity...
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I don't supose it matters much if it's 1 gram in 1000 tonnes or 1 gram in 3000 tonnes.
A few grams in just 42 tonnes is a lot better, but I'm glad I didn't have to do that experiment.
|
|
|
lacrima97
Hazard to Self
 
Posts: 93
Registered: 24-7-2005
Location: MS
Member Is Offline
Mood: experimental
|
|
Does anyone know of any sources of pure cesium?
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
This sounds like it should be in a new thread.
Are you looking for a cesium salt or the metal itself? If you're looking for the metal, look for chemical suppliers (very expensive), element
suppliers (less expensive) and eBay.
|
|
|
lacrima97
Hazard to Self
 
Posts: 93
Registered: 24-7-2005
Location: MS
Member Is Offline
Mood: experimental
|
|
Yeah, I have noticed that the pure metal (what I want) is extremely expensive. I believe that electrolysis of a cesium compound is out of the
question, so I am going to just rid myself of the idea. I have nor the money for the pure metal, nor the stupidity to electrolize such a metal.
By stupidity, I mean with my equipment.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
And what kind of experiments did you have in mind for the elemental cesium? The stuff is so reactive (e.g. explodes in contact with water, inflames in
air), how would you manage to keep it around without causing accidents?
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Store it under mineral oil?
Bromic's patent for making sodium in the 'unconventional sodium' thread also includes an easy synthesis for elemental Cs. Keep in mind though that no
one here has gotten the processes in that patent to work yet. We're still trying to figure it out.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Cs is just so reactive I would likely not feel comforatble working with it at home without an inert gas atmosphere glovebox. Cesium hydroxide is so
corrosive that if your cesium were to react with air or water accidently you would have a nasty spill on your hands.
If I get this summer research scholarship I applied for I will get to play with elemental cesium and other lower alkalis to make alkali mercurides and
aurides. 
|
|
|
olmpiad
Harmless

Posts: 29
Registered: 2-6-2006
Location: this thing known as earth
Member Is Offline
Mood: Dandy
|
|
I did the procedure as first stated, but reduced everything to 10% of the original weights. It actually works quite well, and the Cs-Alum forms very
quickly by placing the solution in a fridge (after about four hours of decanting). Ill post pictures soon. They crystals do not look too much
different than normal alum, but their solubility is a dead give away. First Cs salt I've ever made, and it was quite a success! Ill post pictures
soon.
[Edited on 2/7/07 by olmpiad]
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Where did you get the ore from?
|
|
|
olmpiad
Harmless

Posts: 29
Registered: 2-6-2006
Location: this thing known as earth
Member Is Offline
Mood: Dandy
|
|
Picture: 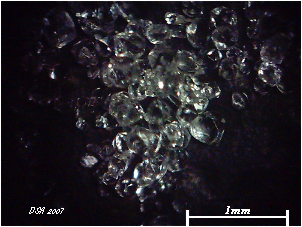
Unionized:
i got the Pollucite from my mineral collection (minerals are my passion), It is from a rare locality in California.
|
|
|
pantone159
National Hazard
   
Posts: 590
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Offline
Mood: desperate for shade
|
|
One link offering to sell pollucite samples, $25...
It doesn't say how much, though.
http://www.sciencemall-usa.com/polcesmin.html
|
|
|
olmpiad
Harmless

Posts: 29
Registered: 2-6-2006
Location: this thing known as earth
Member Is Offline
Mood: Dandy
|
|
I find that quite expensive. The site says that the specimen comes in a Perky Box, which means that it is a TN specimen. As for size reference, this
is known as a Thumbnail to us mineral collectors, which is exactly what it sounds like: the size of your thumbnail. In other words, probably no more
than 5g, and it cost 25$. I paid $7 for my specimen, and it is a C (for those of you who do not collect minerals, this is known as a Cabinet size),
and it weighs a good 500-600g.
|
|
|
| Pages:
1
2 |