Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Purification of perchlorates
I am interested in views and potential procedures for the practical purification of electrolytically produced perchlorate salts, sodium and/or
potassium... preferably something that can be scaled to moderately large size, say 2 to 4 kilos of salt.
In the past, I have used potassium metabisulfite in an acidified liquor of the crude salt to generate SO2, which in turn preferentially reduces the
chlorate ion to chloride. When used in a hot, saturated solution of KClO4 (contaminated with KClO3), the waste products after cleanup are KCl and
K2SO4.
The solution is allowed to cool slowly, then refrigerated. The crystals of KClO4 are harvested, and the unwanted chloride and sulfate salts, still
aqueous, are discarded. A light wash of the perchlorate xtals with ice water, and the product is nicely pure.
The purity of the perchlorate salt was tested as follows:
For chlorate: n-phenylanthranilic acid + sulfuric acid. No chlorate noted.
For chloride: Silver nitrate solution is added dropwise; zero haze or cloudiness observed.
For sulfate: Lead nitrate solution is added; no lead sulfate ppt noted.
The challenge is to minimize the amount of potassium metabisulfite used, and to hopefully generate a procedure that will be repeatable and reliable,
and not require a dozen tests for chlorate before determining when the solution is cleaned up. The first time I tried this, it took over 4 grams of
metabisulfite to clean 20 grams of crude perchlorate, and I have no doubt that it was my poor technique.
Initially, I was adding small portions of the metabisulfite to the hot liquor, but this was flashing to SO2 gas immediately and escaping with almost
no contact with the solution. Eventually, I began to add aqueous metabisulfite using a pipette to the bottom of the perchlorate solution... this
seemed to work much better.
Essentially, I'm looking for suggestions... how to get intimate contact of the SO2 gas with the perchlorate solution. I'm also interested in any
thoughts on other tests for purity for electrolytically produced oxidizer salts, particularly for sulfates. I have confidence in my tests for
chlorate and chloride.
The n-phenylanthranilic acid test does work well, but there may be even simpler ways to do it, as the n-paa is not a cheap or common chemical.
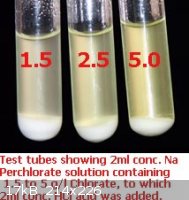
On the left is the NPAA test on 0.1 g/l KClO3 in water. The tube on the right is commercial perchlorate. The NPAA test works well down to 0.01 g/l,
or 10 ppm.

|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
The solubility of SO2 in water drops off a factor of four hundred from 0 °C to 90 °C (see this). Adding it to hot liquor is essentially asking for a gas-liquid reaction rather than one in aqueous solution. If you do it hot, you could
use gear for this kind of reaction. Alternately, you could lower the concentration and do the reaction colder.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
I only attempted to purify perchlorate once to make some perchloric acid. I had run a cell untill the chlorate level was down at approx. 2 grams per
liter. I think I had about 1.5 liters of solution.
I figured out how much Iron Sulphate I needed to destroy the Chlorate present and was very surprised to find that you need 22 grams of FeSO4:10H20 for
every gram of Chlorate. It looks more like contamination of the Perk. solution than purification.
6FeSO4 + NaClO3 + 3H2SO4 ====> 3Fe2(SO4)3 + NaCl + 3H2O
I turned to Sodium Bisulphite (Camden tablets for wine making) You need 2.7 grams bisulphite per gram Chlorate. Assuming the tablets were 50%
Bisulphite I was adding quite an amount of them. I kept getting positive results everytime I tested with indigo carmine. Perhaps my camden tablets had
only a small amount of bisulphite in them. They were very old too. Having to boil/heat the solution is a PITB too.
I got fed up and just made the perchloric acid anyways as the addition of Conc. HCl (to make the acid) was going to destroy the chlorate anyways
giving a yellow (ClO2) solution. I only wanted dilute perk acid so this was not going to be dangerous.
You can got a very quick and dirty test (a titration actually!) for the presence of Chlorate by adding a few ml of your solution to conc. HCl and
seeing how yellow it goes. It's quite a good way to estimate the amount of Chlorate present once you have a standard yellow colour to compare the test
tubes (or whatevery you use) with. You can make a 'standard' colour by adding a known amount of Chlorate to water and using a few ml of this solution
to give you your yellow 'titration standard' colour. You must know how much actual Chlorate you added. (and if it Na or K etc).
There is some details and photos on my page under 'Tests for Chlorate'.
My own opinion that if you are going to get into cleaning Perchlorate then the best way to go it to have a Sulphur Dioxide generator standing by that
burns sulphur. This is easily made. There was a diagram of one on SCIMAD somewhere but I cannot remember where. When finished you can simply
extinguish the sulphur and leave where it is untill the next time it's needed. The SO2 gives minimum contamination. Iron Sulphate, Acids, Bisulphite
(and the other 'solid SO2 generator compounds") leave all sorts of things behind.
Great piece of info. about the solubility of SO2.
Would Hydrogen Sulphide gas destroy Chlorate? Quite a stink no doubt.
Regarding the SO2 generator I would imagine a bladder (big plastic bag) would be a great help. If you were to just leave the SO2 in contact with the
surface of the liquid (and perhaps stirr the solution) overnight, using something like an inverted funnel. It looks like its easy to get rid of the
SO2 that is not used but still dissolved . Just boil the solution. It can be hard to keep the sulphur burning I think in generators sometimes. Will
have a look on the board for the diagram/design someone did (and actually used).
Page seems to be down.
Picture attached:
Dann2
[Edited on 22-6-2012 by dann2]
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Thanks guys...
@ W.F. - This cleanup attempt was made a while ago, and you reminded me of something I did remember, the reverse solubility of the SO2 gas w/regards
to temperature. SODIUM perchlorate would be the ideal subject for an SO2 cleanup, as sufficient amounts dissolve even in cold water. Unfortunately
for potassium, you only get decent solubilities at much higher temps, and of course the SO2 gas is driven from a hot solution. So this would be a
challenge.
I work almost exclusively with potassium salts from start to finish. This has advantages AND disadvantages, and in the case of clean-up, the
disadvantages rear their ugly heads.
As you guys mention, something I need to investigate is an SO2 gas generator to see if a bubbling arrangement might be effective. When a KClO4 batch
is harvested (crude) from a cell, the crystals tend to be pretty massive, which is a good thing from a purity aspect. If these xtals are reasonably
washed, I would guess that their purity would be pretty respectable. Perhaps one washing technique would be ice cold water carrying dissolved SO2
gas, which would reduce any aqueous chlorates clinging to the xtals, followed by pure water to wash away culfates and chlorides.
Obviously there are going to be SOME chlorate solids in the product, and the only way to get rid of these is recrystallization. The solubility of
KClO3 is >> KClO4. If the recrystallization liquor is then allowed to cool, but not refrigerated, the chances that remnant chlorates would
remain dissolved is much higher than if the sample is chilled. More perchlorate would be lost, but of course the aqueous portion of the
recrystallization is good seed stock for additional electrolysis.
Lots to think about. I think the general process to create quality K-perchlorate would be:
1) Collection of xtals. Wash with cold, SO2-saturated water, wash again with pure, cold H2O
2) Combine perchlorate xtals with room temp water of a volume calculated to dissolve the batch at 100 C. Begin heating, slowly, while also bubbling
SO2 gas. As the water heats, the concentration of SO2 diminishes while the concentration of perchlorate rises as the solids dissolve.
3) Once fully dissolved (hopefully hot filtering not necessary), allow slow cooling in an insulated container to promote large xtals.
4) Decant/filter at some temp above zero, keeping any chlorates (if any are still present) and chlorides dissolved
5) Wash xtals with pure ice water; dry. Test the product for chlorate with any test capable of detecting minute impurities. Any residual sulfates or
chlorides should not affect the perchlorate.
6) For extreme purity, additional recrystallizations can be carried out, although I'd skip the use of SO2 in subsequent recrystallizations.
dann2, where are your web pages these days? I like the test you describe using plain HCl. If you'd like to try NPAA, let me know, I can ship you a
few grams. A little goes a very long way.
To Do: Investigate methodologies for controlled generation of SO2 gas, and an apparatus to deliver it to either cold water, or solutions of
chlorate/perchlorate. An interesting test would be to create standardized solutions of chlorate, and calculate what quantities of SO2 would be
needed. If that is known, a sample of "dirty" perchlorate could be quantitatively tested for chlorate, and the correct amount of SO2 generated.
Any other candidate "clean-up" reagents useful for reduction of chlorate to chloride, leaving perchlorate relatively intact?
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
I forgot that you were working with K. I am inclined to think in terms of Na.
My page seems to be down at the moment. I have attached the relevant file. There is nothing in there you did not see before.
Regarding the 'test' using HCl. It's useful really as a ready recknor to find out (roughly) how much Chlorate you have per liter so that you will have
an idea how much chemical to add for destruction of the Chlorate. I would not see it as a substitute for P. acid or Indigo Carmine.
I have both, thanks for the offer.
Dann2
Attachment: destroy.html (9kB)
This file has been downloaded 3034 times
[Edited on 23-6-2012 by dann2]
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Thanks dann2... I enjoy simply pondering this stuff when I'm at work at my "real" job, and cannot mess in the lab.
I sometimes believe we underestimate how pure some of our products can be. I remember analyzing crude, unrecrystallized KClO3 which was simply
harvested directly from a cell, and given a decent wash in the filter paper with ice water. The Xtals were redissolved, and my best investigations
showed them to be 99.3% KClO3 and the remainder KCl. Probably traces of perchlorate as well.
Anyway, I think it is a fun challenge to attempt to work a sample to extreme purity.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Swede  | | More perchlorate would be lost, but of course the aqueous portion of the recrystallization is good seed stock for additional electrolysis.
|
I think this is the core of the right way to think about it. Fractional crystallization processes all favor
recycling of the "waste" liquor into the next feedstock. Similarly, it seems like completely purifying a single batch with minimal batch losses isn't
as productive as getting adequate yield from a single batch and recycling the rest into the next batch. As you point out, "waste" chlorate is actually
feedstock for electrolysis. Fractional crystallization is ideal for extracting the bulk of the chlorate stream.
SO2 purification, given the solubility-crossed relation with K perchlorate, is best suited for removal of small remaining amounts of
chlorate. Even at 1 g / L solubility (pretty hot water), you could still remove, say, a 0.5% chlorate contamination with an aqueous reaction. I
suspect that this is close to the purity you get from a properly controlled fractional crystallization stage, controlled for temperature and
concentration (i.e. specific gravity). That's one of the ways of getting your assay results, to derive it from the process parameters of a
crystallization step.
Perhaps one of the pieces of gear to build is just something that makes saturated SO2 solution at a known temperature. To use it, dilute it
down so that it's vapor pressure is low enough that it doesn't flash off when added to hot liquor.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
The SO2 'reagent' sounds good. I am fairly sure that Sulphurous acid (H2SO3) destroys Chlorate. I always thought that this was made by simply adding
SO2 to water but I have read in another thread (lately) that this is not the case and very little Sulphurous acid forms. How do you make Sulphurous
acid?
One of the ways to make very pure Perchlorates (for lab work) is to make them with Perchloric acid and the carbonate of what you want. But thats not
going to be usable for the garage dude.
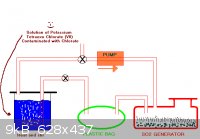
Perhaps a system like in the gif might work. The (big) bag is simple filled with SO2 by burning S and the valves then put the other way so that this
SO2 can be bubbled in for hours.
You would need to be able to stop the SO2 generator. Starvation of Oxygen?
What sort of a getto pump can you use?
I have read in another thread that the secret for the SO2 generator is to have the sulfur on some finly devided inert material so that it will burn
and keep burning. I think the inert material creates a large surface area?
Could Perchloric acid be used (like you use HCl) to destroy the remaining Chlorate. I don't see why not. It is not that hard to make. No contamination
of the Perchlorate to be purified with foreign ions.
Another way to destroy Chlorate is by heating but it does not sound like a good way to proceed IMO. Sound messy.
Dann2
[Edited on 25-6-2012 by dann2]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
You can also react the crude perchlorate product with concentrated hydrochloric acid. This will reduce any traces of chlorate (to chloride and
chlorine gas), while not affecting the perchlorate. The potassium chloride will still need to be removed by fractional crystallization.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AndersHoveland  | | You can also react the crude perchlorate product with concentrated hydrochloric acid. This will reduce any traces of chlorate (to chloride and
chlorine gas), while not affecting the perchlorate. |
Got a citation for that?
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
I did a bit more poking around the other day regarding acceptable purities for KClO4 as used as a pyrotechnic oxidizer. The question was (and
remains) how much chlorate is acceptable in a perchlorate sample, so that the sample can be called perchlorate for use in compositions?
The U.S. military has a MIL-SPEC (military Specifications) document for just about everything, including KClO4. I found it. The document is called
MIL-P-217A and includes two files. The second file is corrections to the first.
MIL SPEC main file
MIL SPEC addendum
The PDF also has quantitative analysis methods for certain contaminants, but nothing we haven't heard of before.
Interestingly, the maximum acceptable quantity of chlorate, as KClO3, for the batch to pass, is 0.10% by weight. That is 1 part in 1,000, (1,000 ppm)
a level of contamination easily detectable by our basic known tests. It is not difficult to clean perchlorate of chlorate to 10 ppm.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Acidified hydrogen peroxide can also be used to reduce chlorate, under certain conditions. (again, the less reactive perchlorate will not be reduced)
Chloric acid does not react with hydrogen peroxide until the temperature reaches 80 °C, at which point it is reduced to HCl and Cl2, with the
liberation of O2.
"Oxidation and Reduction with Hydrogen Peroxide", Wilder D. Bancroft, Nelson F. Murphy, J. Phys. Chem., 1935, 39 (3), pp 377–398
The reaction between solutions of chloric acid (HClO3) and hydrogen peroxide does not have any appreciable reaction rate until a temperatures above 70
°C. Perchlorate is not formed as a reaction product. experiments conducted by Sand, published in Zelt phys. Chem.,50, 465 (year 1904)
Yes, just to confirm, the first reference gives the reaction temperature at 80 degrees and the second reference at 70 degrees.
[Edited on 30-6-2012 by AndersHoveland]
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
So, would you add hydrogen peroxide and HCl to your solution that you are wanting to purify, then raise and hold the temp to 80C? How long do you
need to hold at that temp for the reaction to run to completion? Is this superior to just adding HCl and boiling? That was also one of the ways to
purify on Dann2's website.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Swede  | | Interestingly, the maximum acceptable quantity of chlorate, as KClO3, for the batch to pass, is 0.10% by weight. That is 1 part in 1,000, (1,000 ppm)
a level of contamination easily detectable by our basic known tests. It is not difficult to clean perchlorate of chlorate to 10 ppm.
|
It's a good figure to know, but I want to point out that the military isn't much concerned with color purity
in their devices that use perchlorate.
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
I just don't want the thing to blow up in my face when I have sulfur compounds involved.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by watson.fawkes  | | It's a good figure to know, but I want to point out that the military isn't much concerned with color purity in their devices that use perchlorate.
|
You are correct, and make a solid point, but the question so many of us have is exactly what hyfalcon mentions... At what point can I treat the
perchlorate as a true perchlorate and not a chlorate in terms of compatibility. 0.10% to me seems "impure" but looking at it the other way, it's
99.9% NOT chlorate.
Qualitative tests are always easier than quantitative, but some of the former can be also used to get a rough hack on quantity, especially the color
tests. A standard 0.10% solution of KClO3 can be used for a bit of calibration.
Interesting stuff on the HCl and H2O2. I am going to try and work up some quantites and give it a whirl. It'd be easy to do this on a pretty small
scale by making some stock "dirty" perchlorate, or simply taking small amounts of crude electrolytic KClO4, and seeing what it takes to get the
chlorate down to well below 0.10%.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
From US 2,392,769
Sulphur dioxide does not interfere with this procedure. Sulfur dioxide is therefor added to this solution until a test make with the indigo carmine
indicator shows the presence of less than one part per million of chlorate, that is, the elimination of substantially all of the chlorate and any
other halogen oxy acids.
The patent above seem so implicitely suggest that you need to go to one part per million for to declare the Perchlorate 'free' from Chlorate. They are
making explosives I think.
From above, do some of you think that strong acids (HCl say) will not eliminate ALL traces of Chlorate from Perchlorate?
Don't know myself. I would imagine that Iron Sulphate will emiminate ALL Chlorate.
I would imagine that the bisulphates, metabisulphates and SO2 will eliminate ALL Chlorate as they are all the same as the SO2.
Dann2
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Table here for specs. on Ammonium Perchlorate
The full article is
here.
It shows a Chlorate content of 0.002% with a max. allowable of 0.15% !
I guess there is no sharp cut off point for Chlorate content in Perchlorate, where the Perchlorate will be declared dangerous to the point of not
being usable in conjunction with certain other substances (Sulphur say).
Dann2
Attachment: ampertable.html (5kB)
This file has been downloaded 1014 times
|
|
|
woelen
Super Administrator
        
Posts: 8089
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Quote: Originally posted by dann2  |
From above, do some of you think that strong acids (HCl say) will not eliminate ALL traces of Chlorate from Perchlorate? |
I think that HCl can be used to remove all traces of chlorate, but you need a strong excess amount of the acid, need heating and
need a long time for the reaction. I know from experience that the reaction of chlorate with hydrochloric acid can be sluggish, especially when the
acid is dilute.
Quote: Originally posted by dann2  | Don't know myself. I would imagine that Iron Sulphate will emiminate ALL Chlorate.
I would imagine that the bisulphates, metabisulphates and SO2 will eliminate ALL Chlorate as they are all the same as the SO2.
|
I assume that this is a typo, you mean bisulfites and
metabisulfites instead. This is true at low pH. At medium to high pH, the sulfites and derivatives
thereof will not reduce chlorate to chloride.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Yes, they are typo's.
Two more partial documents on Ammonium Perchlorate specs attached. One of them is similar to what Swede posted except it is Ammonium and not Potassium
Perk.
The other one is from a manufacturer making Am. Perk. in Brazil and they give a figure for Chlorate of 0.02%. Thats 200 parts per million.
Attachment: MIL-A-192B.PDF (33kB)
This file has been downloaded 828 times
Attachment: aeq.pdf (107kB)
This file has been downloaded 878 times
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Wouldn't ammonium be a special case, given the dangers of ammonium chlorate? Sodium or potassium chlorate doesn't have this danger factor.
If I was making a perchlorate via electrolysis with the goal being an ammonium conversion, then the stakes are very much higher vs. someone making
pretty colored stars in a 3" shell.
|
|
|
Texium
|
Thread Moved
20-11-2023 at 12:17 |