| Pages:
1
2 |
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
The russian patent, if you click for more details in scifinder it contains enough of the synthesis to attempt it.
There is also another ref, from an Italian journal in the 30s on the synthesis of dichloroglyoxime, it uses bubbling chlorine into a glyoxime solution
in 10% HCl in in an icebath, and bubbling is continued till the yellow colour persists. Scifinder does not find this ref, it must be found via a
couple iterations of looking up references within a paper.
|
|
|
Axt
National Hazard
   
Posts: 814
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Arr your right, from the patent.
Dichloroglyoxime (HON:CClCCl:NOH), useful as an intermediate for functionalized glyoxime derivs. and also for heterocyclic compds. (no data), is
prepd. from glyoxime (HON:CHCH:NOH) and hydrochloric acid by dosing concd. HCl and perhydrol (H2O2) simultaneously and proportionally with stirring
into a suspension of glyoxime in a concd. aq. soln. of CaCl2 in molar ratios of glyoxime : HCl : H2O2 = 1.0 2.0-2.4) 2.0-2.4) 2.2-2.4), resp., at wt. ratios of the total
amt. of H2O and CaCl2 = (1.2-2.8):1.0 and at 15-25. In examples given, dichloroglyoxime is prepd. in yields of 81-98%. Thus, a suspension of 88
parts (all parts given by wt.) of glyoxime in a soln. of 188 parts CaCl2 in 226 parts H2O is dosed with a soln. of 119 parts CaCl2 in 223 parts 36%
aq. HCl and a soln. of 145 parts CaCl2 in 250 parts 30% aq. H2O2 at 15-25, after which the reaction mixt. is held at 18-20 for 30 min, then filtered
and the crystals obtained are washed with H2O and dried to afford 98% dichloroglyoxime. 2.2-2.4), resp., at wt. ratios of the total
amt. of H2O and CaCl2 = (1.2-2.8):1.0 and at 15-25. In examples given, dichloroglyoxime is prepd. in yields of 81-98%. Thus, a suspension of 88
parts (all parts given by wt.) of glyoxime in a soln. of 188 parts CaCl2 in 226 parts H2O is dosed with a soln. of 119 parts CaCl2 in 223 parts 36%
aq. HCl and a soln. of 145 parts CaCl2 in 250 parts 30% aq. H2O2 at 15-25, after which the reaction mixt. is held at 18-20 for 30 min, then filtered
and the crystals obtained are washed with H2O and dried to afford 98% dichloroglyoxime.
Heres the furazan references again as requested. http://rapidshare.com/files/128717968/furazan-refs.zip.html
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
The ref file is corrupted.
The italian procedure to dichloroglyoxime is: (translated)
5g of glyoxime are dissolved in 150mL of 10% HCl and cooled in an ice bath. Chlorine is bubbled through until the yellow colour just persists and the
solution is allowed to sit 12 during which time the solution crystalizes. Dichloroglyoxime is isolated as white crystals.(nothing crystalized for me!
 ) )
Also, the russian patent dident give the yields they stated on small scale, and its a royal pain saturating everything with calcium chloride. No
confirmation on product identity yet.
[Edited on 11-7-2008 by The_Davster]
[Edited on 19-7-2008 by The_Davster]
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
In this US patent http://www.pat2pdf.org/patents/pat6342589.pdf it states on page 2:
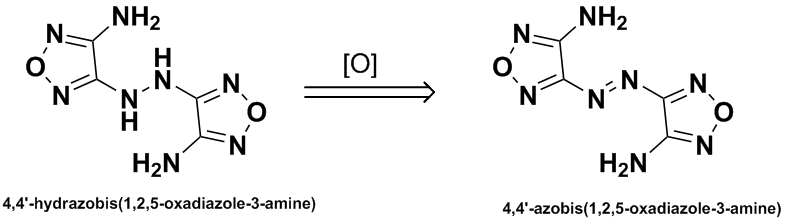
| Quote: | | It was found that 327 kJ/mole of energy was gained in the transformation of 4,4'-hydrazobis(1,2,5-oxadiazol-3-amine) to
4,4'-azobis(1,2,4-oxadazol-3-amine). The latter material is a thermally stable, insensitive explosive. |
No reference to this conversion was given, nor to the preparation of the hydrazo compound. I have been unable to find any references on these points.
Is this compound made from DAF? Or is it made from DAAF via reduction of the N-oxide & azo group?
On page 3 additional interesting info is given on reagents that have been successfully used to oxidize hydrazo functions to azo groups: Br2, MnO2,
HgO, HNO2 & NO2. I'll add Cl2 to that list.
[Edited on 10-8-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Axt
National Hazard
   
Posts: 814
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
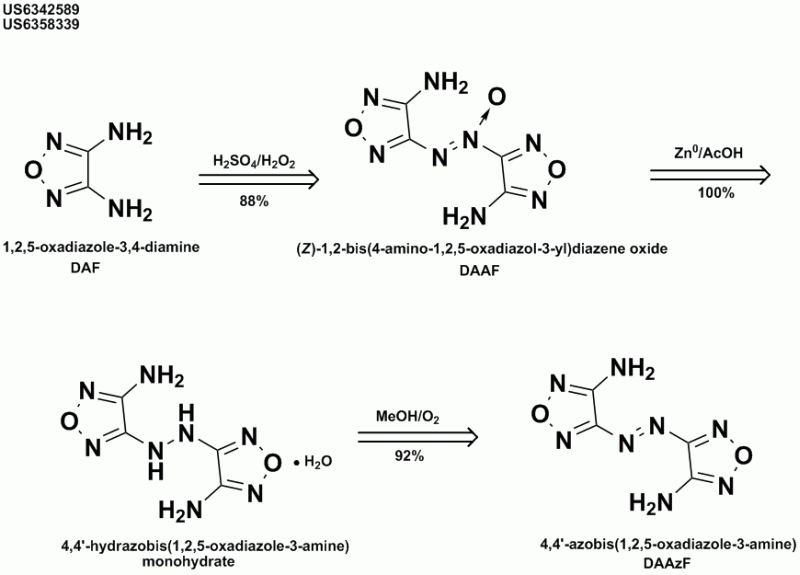
Its preparation is in the DAAF patent I cited. Reduction of DAAF with AcOH-Zn then oxidation with air in methanol.
"The explosive performance of DAAzF was lower in both velocity and pressure as the increase in heat of formation was not sufficient to offset the drop
in oxygen balance compared to the DAAF" US6358339
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Axt
Its preparation is in the DAAF patent I cited. Reduction of DAAF with AcOH-Zn then oxidation with air in methanol.
"The explosive performance of DAAzF was lower in both velocity and pressure as the increase in heat of formation was not sufficient to offset the drop
in oxygen balance compared to the DAAF" US6358339 |
Many thanks!
In the attached ChemDraw graphic I reverted to their hybrid IUPAC/CAS nomenclature because the program diudn't like the 'furazan' nomenclature for
DAAF.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Axt
Its preparation is in the DAAF patent I cited. Reduction of DAAF with AcOH-Zn then oxidation with air in methanol.
"The explosive performance of DAAzF was lower in both velocity and pressure as the increase in heat of formation was not sufficient to offset the drop
in oxygen balance compared to the DAAF" US6358339 |
Many thanks!
In the attached ChemDraw graphic I reverted to their hybrid IUPAC/CAS nomenclature as the program didn't like the 'furazan' system name for DAAF.
[Edited on 10-8-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
Woiuld you have the structure of 1,4,5,8-tetraazadifurazano[3,4-c][3,4-h]decalin? It is produced from 3,4-diaminofurazan, aq HCl & aq glyoxal in
http://www.pat2pdf.org/patents/pat4503229.pdf. I would assume they would form the cyclic bis-Schiff base [1,2,5]oxadiazolo[3,4-b]pyrazine but they
convert this decalin product into the corresponding tetranitro derivative.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
HMX
Harmless

Posts: 1
Registered: 19-12-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Axt
Its preparation is in the DAAF patent I cited. Reduction of DAAF with AcOH-Zn then oxidation with air in methanol.... |
I did it on another: SnCl2 H2O in HCl. Yield is great!
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Axt: In your reference file, you have a file called "furazan derivatives- high energetic materials from diaminofurazan" and it is 148kb. What is the
journal(date, issue, volume too if that is possible) that this is from? I could not match it with any in your reference list.
Thanks
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
It's been a few years since this thread was made. I think it might be good to update it with some newer information. A method for diaminofurazan,
where a yield is described is in US 20090137816 which came out just a few years after the thread. It doesn't use a pressure reactor, but a fluid
medium at atmospheric pressure. DNAF also has an experimentally measured D. of 10.0 km/s at p= 2.02g/cc, this rate being an extrapolated value of the
crystal density from a measured detonation rate (ref.: DOI: 10.1016/j.jhazmat.2004.04.003). Another interesting very powerful compound, related in the
series is the hydroxylamine salt of 3-nitramino-4-nitrofurazan (HANNF). The ammonium salt (ANNF) is also not far behind.
Attachment: US20090137816.pdf (22kB)
This file has been downloaded 1828 times

|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I found an interesting reaction that may be useful for forming azoxy linkages between two energetic molecules, to increase density.
The paper also describes the reaction of phosphine (PH3) with nitrobenzene. There was no reaction at neutral conditions, but when sodium hydroxide was
added then azoxybenzene was produced in high yield. PH3 will also reduce 1-naphthol to naphthalene.
"Phosphine as a Reducing Agent"
SHELDON A. BUCKLER, LOIS DOLL, FRANK K. LIND, MARTIN EPSTEIN. J. Org. Chem., 1962, 27 (3), pp 794–798
So the idea that PH3 could reduce H3PO3 is quite plausible.
Here is a picture showing the structure of azoxybenzene:
http://upload.wikimedia.org/wikipedia/commons/4/4c/Azoxybenz...
C6H5-N=N(O)-C6H5, where the oxygen atom is bonded to a nitrogen atom, not carbon.
[Edited on 7-12-2011 by AndersHoveland]
|
|
|
Explosci
Harmless

Posts: 9
Registered: 20-4-2012
Member Is Offline
Mood: www.explosci.com
|
|
Hi Axt, I used your procedure when making diaminofurazan. Was quicker to google your procedure than look it up in the literature
It gives nice big crystals! I took a photo and it is here: http://www.explosci.com/diaminofurazan-crystals/
|
|
|
Motherload
Hazard to Others
  
Posts: 245
Registered: 12-8-2012
Location: Sewer
Member Is Offline
Mood: Shitty
|
|
Has anyone tried using a stainless steel steam cooker (pressure cooker for lentils and beans etc) with success to condense Hydroxylamine and Glyoxal
to make DAF ?
"Chance favours the prepared mind"
"Fuck It !! We'll do it live !!"
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I have recently attempted to synthesis diaminoglyoxime via both methods outlined by Axt. I got slightly lower yields than reported by Axt but still
reasonable. The only issue is that even after recrystallisation that prepared via the glyoxime intermediate was straw coloured while that prepared
from glyoxal in a single step was almost white. Has anyone else done either preparation and has any comments. I have yet to characterise either
material via melting point but I wondered if the later was mainly glyoxime.

By the way how the hell do you get images to display at a reasonable size instead of a poxy thumbnail. Some posters seem to be able to make their
image fill the available column width?
[Edited on 10-4-2013 by Boffis]
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
This method for the synthesis of diaminofurazan (DAF) is more appropriate for an amateur than the traditional method which uses the pressure reactor,
the method has been used by Shreeve et al. for the synthesis of 3,3'-Dinitroamino-4,4'-azoxyfurazan and Its Derivatives [1]. Dr. Jean'ne M.
Shreeve is a very known scientist in the field of HEDM synthesis. from [1]:
"A mixture of 40% glyoxal (11.5 mL, 100 mmol), hydroxylamine hydrochloride (45 g, 600 mmol), and urea (35g, 580 mmol) in 50 mL of water was added
to sodium hydroxide solution (28 g of NaOH in 60 mL) dropwise. After the addition, the mixture was refluxed for 2 h and then 35 mL of water was
distilled under reduced pressure. The reaction mixture continued to reflux for another 12 h and then cooled to 5 °C. The precipitate was filtered and
crystallized using 50 mL of water to give a pure product as a white solid (4.5 g, 45%)"
what is also an advantage in this method is that there is no need to preform the diaminoglyoxime used in the traditional method. The yield are also in
line with the old method. This new synthetic procedure for DAF was originally published in [2].
References:
[1] Zhang, J.; Shreeve, J. M. “3,3'-Dinitroamino-4,4'-azoxyfurazan and Its Derivatives: An Assembly of Diverse N-O Building Blocks for
High-performance Energetic Materials,” Journal of the American Chemical Society, 2014, 136, 4437-4445.
[2] Ge, Z.-X.; Wang, X.-J.; Jiang, J.; Wang, B.-Z.; Fu, X.-Y. Synthesis of 3,4-dinitrofurazan. Chin. J. Synth. Chem. 2008,
Issue 3, 260.
Dany.
[Edited on 28-6-2014 by Dany]
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Thank you Dany,
These are interesting articles; the synthetic route to DAF without a pressure vessel is very useful. I shall try this in the near future. I have also
just acquired some 50% hydroxylamine solution which simplifies the process further because it avoids the free basing of hydroxylammonium chloride.
Have you ever tried the diaminoglyoxime to DAF via simply heating with glycol as a high temperature solvent as mentioned in one of the patents above?
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I recently tried the preparation of DAF via the direct Chinese route outlined in the references given by Dany above. I used 2/5 of the quantities but
otherwise tried to follow the procedure to the letter. This is easier said than done as after the removal of a proportion of the water a white
ammoniacal smelling sublimate started to accumulate in the reflux condenser. At first I simply pushed it down into the flask with a glass rod but over
time the sublimate became harder and I could no longer dislodge it. After 4 hours refluxing I had to stop the reflux and rinse the condenser, a white
crystalline ppt had started to form by this time. After another 8 hours refluxing I cooled the flask in the fridge and filtered off the now yellowish
heterogeneous product; 2.24g.
I recrystallized it from 18ml of water (very slightly less than the 20ml suggested) and obtained 0.652g of pale straw yellow prisms. Mp determination
gave Mp 207-208 C rather higher than that given for DAF (180-181 C) and closer to, though somewhat higher than, that given for diaminoglyoxime
(200-204 C). I tested the product with ammoniacal nickel acetate solution and obtained a brick red ppt that is characteristic of DAG, I don't know how
DAF would react with this reagent (its the reason I am trying to prepare it!) but in all probability I have simply prepared DAG by a more complex
route!

The crude product; note the heterogeneous nature

The recrystallized product Diaminoglyoxime?
[Edited on 16-1-2015 by Boffis]
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Axt  | Arr your right, from the patent.
Dichloroglyoxime (HON:CClCCl:NOH), useful as an intermediate for functionalized glyoxime derivs. and also for heterocyclic compds. (no data), is
prepd. from glyoxime (HON:CHCH:NOH) and hydrochloric acid by dosing concd. HCl and perhydrol (H2O2) simultaneously and proportionally with stirring
into a suspension of glyoxime in a concd. aq. soln. of CaCl2 in molar ratios of glyoxime : HCl : H2O2 = 1.0 2.0-2.4) 2.0-2.4) 2.2-2.4), resp., at wt. ratios of the total
amt. of H2O and CaCl2 = (1.2-2.8):1.0 and at 15-25. In examples given, dichloroglyoxime is prepd. in yields of 81-98%. Thus, a suspension of 88
parts (all parts given by wt.) of glyoxime in a soln. of 188 parts CaCl2 in 226 parts H2O is dosed with a soln. of 119 parts CaCl2 in 223 parts 36%
aq. HCl and a soln. of 145 parts CaCl2 in 250 parts 30% aq. H2O2 at 15-25, after which the reaction mixt. is held at 18-20 for 30 min, then filtered
and the crystals obtained are washed with H2O and dried to afford 98% dichloroglyoxime. 2.2-2.4), resp., at wt. ratios of the total
amt. of H2O and CaCl2 = (1.2-2.8):1.0 and at 15-25. In examples given, dichloroglyoxime is prepd. in yields of 81-98%. Thus, a suspension of 88
parts (all parts given by wt.) of glyoxime in a soln. of 188 parts CaCl2 in 226 parts H2O is dosed with a soln. of 119 parts CaCl2 in 223 parts 36%
aq. HCl and a soln. of 145 parts CaCl2 in 250 parts 30% aq. H2O2 at 15-25, after which the reaction mixt. is held at 18-20 for 30 min, then filtered
and the crystals obtained are washed with H2O and dried to afford 98% dichloroglyoxime.
Heres the furazan references again as requested. http://rapidshare.com/files/128717968/furazan-refs.zip.html |
Rather than start a new thread I thought I'd try to resurrect this interesting thread again!
While rummaging through the technical literature looking for information on my pet project (fulminuric acids) I came across an interesting paper that
gives a detailed preparation for dichloroglyoxime and its use in the synthesis of 3,3-bisisoxazoles (1). These later compounds are useful ligands of
the N=C-C=N (2,2-bipyrydyl and 1,10 phenanthroline type) that form luminescent complexes with some heavier transition metal.
The preparation basically involves reacting glyoxime with N-chlorosuccinimide in DMF at room temp, dilute with water and extract with ether. The yield
is reportedly good (91%). If you have access to DMF this should be possible. The whole reaction scheme is remarkably basic given the date of the paper
(2013). The ref. is:
(1) van dr Peet et al.;
J. Org. Chem.; 2013; v78, is14; pp7298-7304;
DOI is - jo4008755
It may be possible to replace the DMF with other aprotic solvents. The main issue is that when the reaction solvent is diluted with water the
resultant should be immiscible with ether. THF may be a possible substitute for ether as it is becoming more accessible due to its us in 3D printing.
|
|
|
| Pages:
1
2 |