IndependentBoffin
Hazard to Others
  
Posts: 150
Registered: 15-4-2011
Member Is Offline
Mood: No Mood
|
|
Review of methods to make nanoscale powders
Apologies if this is in the wrong forum but can anyone suggest some references that provide a review of the different manufacturing techniques for
making nanoscale powders?
I am particularly interested in:
1) Nanoscale reactive powders - e.g. metals, organic compounds
2) Production capacity estimates
3) Figures for cost per gram
4) Comparison of how the production method, capacity and cost increase as you go from micrometric to nanometric powders
5) Methods that have been tried but were unsuccessful
I am new to the field and just want more bedtime reading 
I've tried searching for "review nano powder manufacture" on Google Scholar but it just turns up methods for making specific powders rather than a
more general introduction.
|
|
|
condennnsa
Hazard to Others
  
Posts: 217
Registered: 20-4-2010
Location: Romania
Member Is Offline
Mood: No Mood
|
|
I was also wondering about this, more particularly about the possibility of an amateur to make nanopowders by using a planetary ball mill.
Wikipedia says that a high quality ball mill is capable of producing particles as small as 5 nm.
also on the wiki nanoparticle article , : "There are several methods for creating nanoparticles, including both attrition[disambiguation needed] and
pyrolysis. In attrition, macro or micro scale particles are ground in a ball mill, a planetary ball mill, or other size reducing mechanism. The
resulting particles are air classified to recover nanoparticles"
[Edited on 22-4-2011 by condennnsa]
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Im sorry I can't give much help but perhaps what I remember could give you a hint into the direction to look. I remember reading something....
somewhere.... that spoke of nano scale metal particals being formed thru the precipitation of a metal while using some kind of surfactant to assure
that the precipitated metal powders did not conglomerate into larger clusters.
I may have even posted the paper somewhere here but im sorry that my memory fails me quite often as of late. Its quite possible in the defunct ORMUS
thread from a few years ago but im not sure.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
IndependentBoffin
Hazard to Others
  
Posts: 150
Registered: 15-4-2011
Member Is Offline
Mood: No Mood
|
|
It would have to be in an inert atmosphere if you're going to use a ball mill to make nanoscale powder. Fe, Cu and Ni powder for example spontaneously
ignite below around 10 - 30nm, W at 1 micron and Zr and Na at 10 microns, c.f. "Review of Zirconium-Zircaloy Pyrophoricity," Cooper, T. D. (1984) that
is attached. Anything in the nanometer range will probably react so quickly it is liable to explode if there are large quantities of it.
It would be interesting to see if there is a graph of ball mill run time and the statistical particle size distribution. It seems to me that there
would be, say, a normal distribution of particle sizes. Particles in the nm range would probably be at the tail end of the normal distribution.
Running the ball mill in anything but an inert atmosphere (nitrogen and CO2 probably don't count - you need a noble gas) will probably cause the
immediate oxidation of nanometric reactive particles.
Attachment: review_zirconium_zircaloy_pyrophoricity.pdf (953kB)
This file has been downloaded 1329 times
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
I would be shocked if anything produced in a ball mill would be considered Nano but I could be wrong. I would look more into precipitated metals more
then anything. With the right ligin the are able to reach increadibly small scale particals muc smaller then a ball mill could ever achieve,
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
condennnsa
Hazard to Others
  
Posts: 217
Registered: 20-4-2010
Location: Romania
Member Is Offline
Mood: No Mood
|
|
Here's an interesting article I found dealing with this:
http://cbe.tulane.edu/faculty/mitchell/JournalArticles/Nanop...
[Edited on 23-4-2011 by condennnsa]
|
|
|
IndependentBoffin
Hazard to Others
  
Posts: 150
Registered: 15-4-2011
Member Is Offline
Mood: No Mood
|
|
Thank you, I am reading it now 
I do recall a method of making nanoscale powders that involved sending an electric pulse through a wire suspended in an inert gas. The wire vapourises
and nanoscale powder is produced.
Obviously not an ideal method if you want kgs of the stuff!
|
|
|
condennnsa
Hazard to Others
  
Posts: 217
Registered: 20-4-2010
Location: Romania
Member Is Offline
Mood: No Mood
|
|
Yeah, it's really interesting, as it points out that these high energy mills are well capable of going all the way to nano. The major issue is the
contamination.
Look at the graph where they milled aluminum powder to test contamination.
With stainless steel jar and media the %SS in the powder was 0.5% after 100 minutes and 1.5% after 1000.
While by using tungsten carbide jar and media contamination was 5% after 100min and a wobbling 35% after 1000! . It seems that harder isn't always
better, as the WC really likes to chew itself up.
I've been checking out prices of these machines and it seems they go from $2000 and up  . .
But they shouldn't be too hard to build , the hardest part would be the planetary gear system, for which a machine shop is a must.
Some more stuff I found online:
They make 39-96 nm Al-Cu particles :
Attachment: 0312223627.pdf (271kB)
This file has been downloaded 640 times
An excellent paper with lots of graphs and pictures, making nanoparticles for use in nanofluids:
http://ethesis.nitrkl.ac.in/192/1/final_report.pdf
[Edited on 23-4-2011 by condennnsa]
|
|
|
IndependentBoffin
Hazard to Others
  
Posts: 150
Registered: 15-4-2011
Member Is Offline
Mood: No Mood
|
|
I have access to a few machine shops that can do contract work. Their rates are £25/hour for manual operated machines and £35/hour for CNC work.
Sorry I can't do it for free as they are subcontractors to me and I have to pass on the costs.
I have a few questions after reading your reference:
1) Does the microstructure of the metal to be nanonised (c.f. micronised) start with make a difference? E.g. a temper-quench specimen with a very fine
grain structure vs. a wholly annealed specimen with a large grain structure?
2) In the oxide dispersion strengthened alloys described on Pg 2, does the manufacture process involve a sintering or hot isotactic pressing phase to
consolidate the powder?
3) On Pg 4 Figure 3, what is the cause of the initial rise in milling time as particle size increases? I would have thought that particle size is
always inversely proportional to milling time.
4) WC probably contaminates the product more than stainless steel because it has a lower toughness. At the point of impact the stresses probably
exceed the elastic limit of most materials and the impact energy must be dissipated by surface energy (crushing/cracking and opening up new surfaces),
plastic deformation (lacking in WC - hence cracking dominates), elastic waves (vibrations, noise) and heat.
[Edited on 23-4-2011 by IndependentBoffin]
|
|
|
condennnsa
Hazard to Others
  
Posts: 217
Registered: 20-4-2010
Location: Romania
Member Is Offline
Mood: No Mood
|
|
Well this is just off the top of my head, after all, everything I know about this is what I've read here and there on the web.
1) I figure that a quenched metal would decrease more rapidly in size in the first period of the milling time, because it will fracture by breaking
apart. While an annealed piece, being softer and more ductile, would have to be bent over and over, which will cause it to eventually break apart to
smaller pieces, so I figure that it will decrease in size more slowly.
2) I don't know I guess so. I guess so.
3) Well I'm as surpised as you, and I didn't notice that myself.
I really don't have an explanation. Maybe it has to do with figure 2 on page 3, some kind of agglomeration effect. I think this is also related to the
material ductility, as until the particles acquire sufficient fatigue in order to break apart, they can agglomerate. Therefore, I would not expect
such a graph for brittle materials.
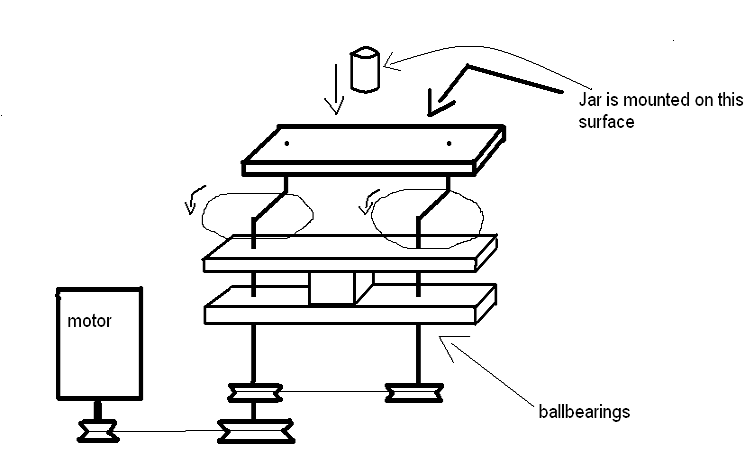
Here is a contraption I've come up with for a high energy ball mill design which does not use planetary gears.
Hope my paint sketch is clear enough, it's basically a system which allows to rotate the jar while eliminating its movement of revolution, thus
creating high centrifugal forces on the walls. Its actually the same as a planetary system, but less efficient since in a planetary mill the rotating
and revolving directions are actually opposite.
[Edited on 23-4-2011 by condennnsa]
[Edited on 23-4-2011 by condennnsa]
|
|
|
IndependentBoffin
Hazard to Others
  
Posts: 150
Registered: 15-4-2011
Member Is Offline
Mood: No Mood
|
|
Nice idea, but I don't think it will last very long from the off-axis forces. There are just so many points of mechanical failure: bending of your
rods, widening of the holes on the plate, cutting through of the rods by the plates, etc. Imagine spinning that at 300 RPM!
Also the structure is fairly complex from a dynamic point of view. There are many possible modes of vibration and we can not guarantee that from your
operating RPMs no modes at all will be excited to resonance. Unless you make the parts out of very stiff materials (natural frequency is proportional
to sqrt(E/density)) you would certainly get low frequency resonant modes.
The advantages of the planetary ball mill is that the components are structurally strong and simple. Therefore they are easy to keep from being
stressed excessively and the design kept away any resonant modes of vibration.
Frankly I do not think it will cost USD$2000 to build your own planetary ball mill professionally if you pay a workshop to do it. Say 1 day's work (8h
@ USD$25 = USD$200), scavenged motorcycle or car engine (you can buy very old ones second hand cars for say USD$300 and other parts USD$500 at most.
So it would come up to about USD$1000 and I am being very generous on the figures here. A CNC machine will cut out the gear teeth in no time (like 1h
@ USD$35), I've seen really old Fords on sale for USD$50 and you can ask for off-cut stainless steel from steel stockholders. If you're lucky and
shrewd about things you can get everything done for USD$500, I think.
|
|
|
condennnsa
Hazard to Others
  
Posts: 217
Registered: 20-4-2010
Location: Romania
Member Is Offline
Mood: No Mood
|
|
Yeah you're right, it was only a sketch though to illustrate the mechanism.
With some math and adjustable counterweights i think it could be made to work... like so
I am really tempted to try to build something like this, to see how it'd work for blackpowder. I remember reading somewhere that high energy mills
decrease the mill time for BP by an order of magnitude compared to conventional ball milling.
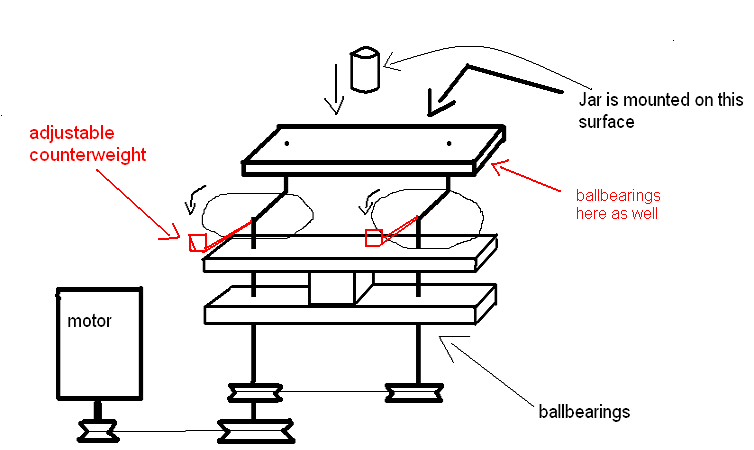
[Edited on 23-4-2011 by condennnsa]
|
|
|
IndependentBoffin
Hazard to Others
  
Posts: 150
Registered: 15-4-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by condennnsa  | Yeah you're right, it was only a sketch though to illustrate the mechanism.
With some math and adjustable counterweights i think it could be made to work... like so
I am really tempted to try to build something like this, to see how it'd work for blackpowder. I remember reading somewhere that high energy mills
decrease the mill time for BP by an order of magnitude compared to conventional ball milling.
|
Yes this is starting to be more promising  . You could even mount another jar
symetrically so that the off-axis forces cancel out. . You could even mount another jar
symetrically so that the off-axis forces cancel out.
Why do you need a ball mill to make black powder? You can already get saltpetre and sulphur in powder form.
Charcoal powder can be bought off Ebay or you can try activated charcoal for water filtration. I think even a plain ball mill will be enough to break
up the activated charcoal to a fine enough powder.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by IndependentBoffin  | | Frankly I do not think it will cost USD$2000 to build your own planetary ball mill professionally if you pay a workshop to do it. Say 1 day's work (8h
@ USD$25 = USD$200), scavenged motorcycle or car engine (you can buy very old ones second hand cars for say USD$300 and other parts USD$500 at most.
|
If you're looking to scavenge parts, there are planetary gear systems in automatic transmissions.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
You forgot plasma, which is one of the most promising possibilities in my opinion. I really dig the no moving parts possibility.
www.kona.or.jp/search/25_039.pdf
http://www.sciencedirect.com/science/article/pii/S0167577X05...
joam.inoe.ro/download.php?idu=2355
http://www.springerlink.com/content/k41432716r85416v/
US patent 6689192
I'm curious to see if (possibly pyrophoric) nanopowders could be useful for sensitizing hydrocarbons used for FAE purposes. Potentially you could even
make a single event FAE with pyrophoric nanopowders, and the delay could be tuned by adjusting the size and/or concentration of the nanopowder.
I have searched and come up with nothing about nanomaterials in FAEs. Maybe it's all classified?
[Edited on 28-8-2011 by 497]
|
|
|
HellstormOP
Hazard to Self
 
Posts: 75
Registered: 13-8-2011
Member Is Offline
Mood: No Mood
|
|
Regarding planetary ball mills, I have good news. Together with one of my friends, who has a machine shop, I'm already working on one. The material is
mostly purchased, the major part of the sketches is made, we also have blueprints of some parts. Our system won't need big gears, we will use smaller
gears, connected by a drive belt and held in place by an aluminium bar. The jar will be fixed on both sides instead of only one, to increase the
stability. I think we should be able to finish it within about 1.5 months. I also will upload some of the sketches I made for it as soon as I can.
[Edited on 28-8-2011 by HellstormOP]
|
|
|
jimwig
Hazard to Others
  
Posts: 215
Registered: 17-5-2003
Location: the sunny south
Member Is Offline
Mood: No Mood
|
|
patents
seems like the patents might give some sort of direction
but the term dynamic gas-phase condensation method pops out right away.... as mentioned previously grinding just doesn't seem to
approach the magnitude of production.
[Edited on 29-8-2011 by jimwig]
craZy jiM wGGns
--packrat, professional bum. -- once just tired
now REtired.
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Quote: Originally posted by IndependentBoffin  |
Why do you need a ball mill to make black powder? You can already get saltpetre and sulphur in powder form.
Charcoal powder can be bought off Ebay or you can try activated charcoal for water filtration. I think even a plain ball mill will be enough to break
up the activated charcoal to a fine enough powder. |
Just mixing finely divided carbon sulfur, and KNO<sub>3</sub> will not make a good black powder. It is necessary to ball mill, or at least
pulverize in a mortar and pedestal for long hours to get individual particles of sulfur and oxidizer to become impregnated in the pores of the carbon.
[Edited on 30-8-2011 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bot0nist  | Just mixing finely divided carbon sulfur, and KNO<sub>3</sub> will not make a good black powder. It is necessary to ball mill, or at
least pulverize in a mortar and pedestal for long hours to get individual particles of sulfur and oxidizer to become impregnated in the pores of the
carbon.
|
It needs to be soaked in water, then dried. This is so that microcrystals of KNO3 can form around the particles of charcoal. If it is not soaked in
water, it will be difficult to get the composition to explode.
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|