GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
Citric acid -> mesaconic acid.
I'm working with citric acid at the moment and trying to get (eventually) mesaconic acid.
Here is my progress:
Citric acid is added to DMF and Xylenes and refluxed for 24 hours. A claisen adapter is added to remove water from the reflux. Color changes occur
within an hour as a yellow forms. After 24 hours the solution is usually slightly darker than previously.
This refluxing causes the loss of water from citric acid to make the itaconic anhydride, which isomerizes to citric acid anhydride, and is then opened
to citraconic, which finally isomerizes to the trans-configured mesaconaic acid.
My problem is yields. I get the product, but not much, and I'm wondering if anyone wants to delve into this problem as it's bugging the fuck outta me.
My work up is simply vacuum distillation. First comes off xylenes, then DMF, then the product under high vacuum. I usually get around 20-30% yields,
and that still has quite a bit of DMF in it.
So - I challenge you all. I've read all the literature there is on this, and it is all excrutiatingly old and unreliable (98% yield, 99% yield on
everything).
I will try this again in dietheylacetoamide (higher B.P. than the MP of citric acid). and let it run for a 1-2 hours.
I will also try DMF/Xylenes for 1-2 hours, and finally benzene for 48 hours.
Question one is - do you guys think this reaction (due to its first order kinetics) would go to completion <24 hours given a reflux at ~120-130°C?
Question two is - ... what do i do 
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
I am unfamiliar with the reaction. Do you not have some online references?
Your yield will be constrained by side reactions, and these may increase with increased temperature or time. It isn't possible to say much on a
purely theoretical basis without knowledge of the mechanism.
If you cannot find a published optimised protocol and you really do want to optimise the yield then you will have to investigate it yourself with a
number of time series at differing temperatures and reaction mixes. It hardly seems worthwhile given the ready availability of the starting material.
A more detailed writeup, with photographs, would be much appreciated.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Looks more like it dehydrates to yield a mixture of cis/trans aconitic acid which then decarboxylates via 6 membered ring with the double bond
(particularly easy decarboxylation) to yield mesaconic acid (and a host of others, including itaconic acid, which is isomeric). Just heat it, in a
high-boiling solvent (or to melt); it will self-catalyze. Add a trace of sulfuric acid, if you find it's too slow.
Perhaps the mechanism is different in DMF?
Check out Pfizer, they were making itaconic acid from citric (made in bulk via fermentation).
See: Hass, H.B. (1955). Itaconic Acid Offers Unusual Promise. Sugar 50 (5), pp. 40-41.
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
Thanks Ozone, I will look that up in a bit.
The reaction mechanism is a bit hard to draw with arrow pushing - alot is going on, but what is basically happening is you are dehydrating a ring;
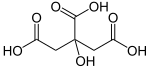
notice the top right of the molecule - you can easily visualize the formation of a 5-membered cyclic anhydride.
Then what happens is you decarboxylate the 'left' (arbitrary) hand carboxylic group, and kick off the -OH group, anti-periplanar to it, creating a
terminal alkene.
This is itaconic anhydride. Itaconic anhydride can further undergo reduction to the di-carboxylic acid, isomerize to the citraconic acid, and finally
it undergoes another isomerization, to the trans-carboxylic mesaconic acid.
The purity of the end product is nearly selective for mesaconic acid - it is kinetically easily to push the reaction to its formation, however,
degradation occurs (not much itaconic or citraconic is found).
The problem is only degredation, not pushing equilibriums or not. I guess I will have to try some more variations in solvent / time.
I would imagine either;
Benzene (lower B.P.) + longer reflux
Or
Done in diethylacetoamide and a [VERY] high boiling reflux agent + short reflux (hour or two).
Perhaps degredation is occuring during the formation of itaconic anhydride - a terminal alkene in the presence of many carbonyl and anionic
carboxylates... Hmmm.
I think mostly this was best that I just write out my thoughts 
|
|
|
|