| Pages:
1
2
3
4
..
19 |
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
| Quote: | | So far no one has explained why the alcohol has to be a tertiary one |
Nicodem offered what sounded like a pretty good explanation somewhere in this thread.
(edit): actually it was in the potassium thread, not this one.
[Edited on 31-12-2010 by bbartlog]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Another source of middling length carboxylic acids is 'green' weed control products. Some of these use C8-C12 straight chain acids, with n-nonanoic
(pelargonic) acid being the most effective and thus favored in products. Generally the acid(s) is mixed with some hydrocarbon solvent, add NaHCO3 or
Na2CO3 solution to tie up all the acid as the salt, then steam distilling off the hydrocarbons (the free acids are pretty goat-like smelly, keeping
them as the salt until needed is not a bad idea).
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by not_important  | Another source of middling length carboxylic acids is 'green' weed control products. Some of these use C8-C12 straight chain acids, with n-nonanoic
(pelargonic) acid being the most effective and thus favored in products. Generally the acid(s) is mixed with some hydrocarbon solvent, add NaHCO3 or
Na2CO3 solution to tie up all the acid as the salt, then steam distilling off the hydrocarbons (the free acids are pretty goat-like smelly, keeping
them as the salt until needed is not a bad idea).
|
Any trademarks spring to mind? These would be about the right length IMHO and could be easily esterified back to methyl or ethyl esters.
[Edited on 31-12-2010 by blogfast25]
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
While experimentation with sodium/potassium production isn't really my thing; I would not mind making these tertiary alcohols for others to experiment
with (i'd actually enjoy making a product for a specific purpose).
I think grignard is not a good way to do this, because, its a pretty expensive and time consuming process, when you consider large volumes of ether,
dry solvents, preparing alkyl halides etc. I have thought of some practical alternatives.
1. BHT. While BHT is not a tertiary alcohol, it is a phenol, so no posibility of alpha hydride elimination. Hopefully it will not be metallated at
any position other than the OH group. Once the OH group is deprotanated the protons of the ring become pretty basic. BHT is available for less than
$15 a pound and should be very fat soluble.
2. Pinnacol coupling of a long chain ketone. This will form a diol composed of 2 tertiary alcohols.
3. Isomerize a branched readily available secondary alcohol to a tertiary alcohol.
4. Oxidize a branched paraffin to an alcohol. This can be done with KMnO4 under certain conditions (do a patent, search isobutane to t-butanol, look
at earlier patents)
I think the ones to focus on are 1 and 3. Option 1 because BHT is directly OTC and might work. Option 3 because I have an easy and cheap method in
mind starting from menthol. In theory, 2 is good and easy but the chain lengths will need to be very long, because per molecule you will have two
alcohols that will be deprotonated. 4 seems good on paper, but I just can't think of a pure source of branched parafins. Also, like the pinnacol
problem, multiple branches would lead to polyols, reducing solubility.
Therefore, I propose BHT be researched and also 1-isopropyl-4-methyl-cyclohexanol, which can be made in just a couple of steps from menthol. A scheme
is attached below (I haven't bothered to show stereochemistry), I haven't worked out the best method for hydration of the olefin, but It is something
I can definitely work out.
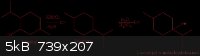
[Edited on 12-31-2010 by smuv]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Search for "fatty acid weed control". Safer's Superfast and/or Topgun Weed Control, SCYTHE (almost 60% pelargonic) , and Weed-Aside, are ones that I
remember but there I'm sure there are others.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by smuv  | (big snip)...
4. Oxidize a branched paraffin to an alcohol. This can be done with KMnO4 under certain conditions (do a patent, search isobutane to t-butanol, look
at earlier patents)
... |
Also bromination of paraffins under fairly mild conditions where the hydrogen reactive will be tert > sec > primary. Do it under fairly mild
conditions, treat the reaction mix with cold NaHCO3 solution (to reduce the amount of elimination) to get the alcohols.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@not_important:
Thanks for both these posts. Read and noted… Petroleum jelly (Vaseline) is probably full of branched paraffins. Not sure how you go about
brominating that though...
@smuv:
Agreed of course on the Grignard reaction as being difficult and fairly wasteful. At some point we’re going to have to look into catalyst recovery
which would make that less important but for now we need to take into account that even small amounts of a target alcohol (which might then prove not
to work!) are resourceful..
All of your ideas are appealing, especially as we may have to look at alcohols with cyclic/aromatic functionality. But as this is a ‘labour of
love’ we’ll also need some ‘division of labour’…
Personally I’ll probably pursue the isopropyl myristate to 2-methyl-2-pentadecanol route because it will give me a ‘Grignard experience'
and because the alcohol should work in highly paraffinic solvents and may be recoverable/reusable from it…
BTW: what software do you use to depict those wonderfully clear structural formulas?
[Edited on 1-1-2011 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Another possible and very OTC precursor may be naphthalene (moth balls). Nurdrage got very good results by using Tetralin, the naphthalene derivative,
as a solvent for K production. This may be due to higher alcohol solubility in Tetralin as opposed to in more paraffinic solvents. This could possibly
be taken advantage of by means of naphthalenic functionality in the t-alcohol.
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
I use chemdraw, actually IMO the image isn't that clear because I didn't put much effort into resizing the image with a photo editor.
I don't think naphthalene is a good idea because naphthalenes are generally easier to metallate and reduce than phenyl derivatives. While tetralin is
a naphthalene derivative it has no naphthalene ring. Therefore in terms of acidity and ease of oxidation tetralin is more similar to a dialkyl
benzene derivative.
About getting the best use per gram of (pre-)catalyst, I think instead of catalyst recovery, effort should be made to make a semi-continuous batch
process. I see it going like this.
1. Dehydrate KOH by heating with Mg as in the first part of the patent. Either allow the solution to settle and siphon off the bulk of the solvent or
vac filter the solution. Save this solvent, it will be reused as "dehydration solvent".
2. Add the dehydrated KOH and Mg to another solution, the "reduction solution". The reduction solution is a mixture of the solvent and pre-catalyst
you are using. Heat this up as usual and reduce the potassium hydroxide. When the run is done, filter, to recover the "reduction solution" (and
potassium).
3. Repeat the process with a new batch, re-using the recovered "dehydration solvent" and "reduction solution".
This of course will only work with an alcohol that is not appreciably lost at the reaction temps.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
On the naphthalene I was simply thinking it might be a cheap and easy precursor. Surely you wiz kids can crack up one of the resonance rings and graft
a 2-methyl-2-ol on there? ;-)
Regarding recovery/reuse of the solvent, assuming indeed the t-alcohol is the catalyst and that none of it gets lost through degradation or
evaporation, then at the end of the reaction when all Mg has been used up the alcohol should be in the dissolved potassium alkoxide form. Separate the
liquid from the solids and it should strictly speaking be reusable as such: adding fresh KOH (with water contained in it) and Mg should hydrolyse the
K alkoxide wholly or partly to KOH + alcohol, the drying process then continues via Mg + H2O and after that the reduction reaction should proceed. No
one has yet attempted to recycle the solvent + catalyst in that way.
--------------------------
Looking at the availability of longer chain precursors for 2-methyl-2-alkanols by Grignard, the choice is basically between 2-ketones and methyl or
ethyl alkanoates, the latter requiring twice the amount of methyl magnesium iodide (a serious cost factor because of the iodine).
Of the equivalent 2-ketones and methyl or ethyl alkanoates the ketones are more expensive. And the cost of any of these precursor (at least going by
Sigma-Aldrich [SA] and similar) rises almost exponentially with chain length. From about C6 the price starts getting expressed in gram amounts, rather
than litre amounts.
The most affordable are:
2-pentanone – methyl propyl ketone – MPK (SA: £23.50/L),
2-hexanone – methyl butyl ketone – MBK (SA: £30.50/50 gram and
4-methyl-2-pentanone – methyl isobutyl ketone MIBK (SA: £32.80/L)
2-octanone – methyl hexyl ketone – MHK (SA: £22.10/500 gram)
Higher ketones become seriously expensive: £29.60/100 gram from MPbio.com for methyl nonyl ketone(MNK) – 2-undecanone. This MNK is also an active
ingredient in cat/dog repellent products like ‘Get off my garden’ sprays and granules but the active ingredient appears to be about 2.5 g/500 g.
2-octanone being a slight exception to the rule: cheaper than 2-hexanone (?!?)
MIBK would be a precursor for 2,4-dimethyl-2-pentanol with an estimated BP of about 140 – 150C. But it’s not much longer than the working
2-methyl-2-butanol (t-amyl alcohol, BP ≈ 100C).
MHK would be a precursor for 2-methyl-2-octanol, about the right length, methinks. (1-) octanol has a BP of 195C, so we should also be in the right
ball park on volatility…
[Edited on 2-1-2011 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Another seriously OTC candidate for a precursor of longer chain 2-methyl-2-alkanols is… fractionated coconut oil!
Acc. Wiki:
Fractionated coconut oil is a fraction of the whole oil, in which the long-chain fatty acids are removed so that only medium chain saturated fatty
acids remain. Lauric acid, a 12 carbon chain fatty acid, is often removed as well because of its high value for industrial and medical purposes.
Fractionated coconut oil may also be referred to as caprylic/capric triglyceride oil or medium chain triglyceride (MCT) oil because it is primarily
the medium chain caprylic (8 carbons) and capric (10 carbons) acids that make up the bulk of the oil.
Being glycerol esters this oil could be Grignarded straight to the t-alkanols or hydrolysed to the acids for work up (caproic (C6) and caprylic acid
(C8) are both soluble in methanol, 7.98 and 6.31 M, capric acid is soluble in alcohol and ether).
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I guess the most practical t-alcohol to chose as a target would be 2-methylhexan-2-ol which can be made from n-butyl bromide and acetone via grignard
addition:

JACS, 51, 1483 (1929) (attached + a later related paper)
n-Butyl bromide can be made from the fairly easily obtainable n-butanol with the NaBr/H2SO4 approach as described on this forum elsewhere on various
lower alcohols.
I could not find any preparative synthesis of fatty t-alcohols from triglycerides, but there are a few articles where there is described that the
treatment of triglycerides with alkylmagnesium gives the corresponding t-alcohols. For example, here is the CA abstract of one such paper where the
use of the reaction for analytical purposes is discussed:
| Quote: | DOI: 10.1007/BF02534006
Rapid analysis of jojoba wax fatty acids and alcohols after derivatization using Grignard reagents.
Pina, M.; Pioch, D.; Graille, J. Dep. Oleagineux, Cent. Coop. Int. Rech. Agron. Dev., Montpellier, Fr.
Lipids (1987), 22(5), 358-61. CODEN: LPDSAP ISSN: 0024-4201. Journal written in English. CAN 107:42039 AN 1987:442039 CAPLUS
Abstract: The title method, based on the action of Grignard reagents, differed from methods previously described, which were essentially based on wax
alcoholysis. Grignard reagents, esp. MgEtBr, reacted on ester functions to turn the wax constituents into primary and tertiary alcs., the latter
being the fatty acid derivs. The mixt. of these alcs. was analyzed by a single gas chromatog. injection. The overall time, .apprx.1.5 h, made this
method suitable for routine anal. It could be also considered for analyzing low-carbon-condensation org. acid esters. |
Attachment: t-alcohols via grignard reactions.pdf (352kB)
This file has been downloaded 1632 times
Attachment: t-alcohols via grignard addition on acetone.pdf (574kB)
This file has been downloaded 1818 times
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
OK. Has anyone here ever heard anything about Barbier-Gringnard reactions? Alkyl halide, zinc dust, water as solvent? It's not exactly rocket
science....
Also kind of a hot topic for "Green" chemistry.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Nicodem:
Interesting how it would seem possible to directly Grignard the triglycerides. But it does cost about 6 mol of GR per mol of triglyceride and needs
the expensive CH3I as a source of alkyl.
The reactions using acetone as a substrate are far more attractive in that respect, thanks for both *.pdfs. Acetone is dirt cheap and easy to dry. For
the 2-methyl-2-alkanols, the precursors would be respectively 1-chloro(butane, pentane, hexane, heptane, octane etc) and these are relatively
affordable [cough!], going by Sigma-Aldrich:
Per 100 ml: C4 = £26.8, C6 = £19.00, C7 = £29.20, C8 = £17.30.
The butyl chloride could be made cheaper by chlorinating n-butanol (£15.30/500 ML) but I think the resulting alcohol may still be a bit short (a C6).
Also 1-hexanol (£8.20/100 ML) isn’t too bad and the resulting C8 alcohol would be in the right BP area.
As ‘natural’, OTC precursors for C6-C8 ols, some essential oils may be considered: citronella oil contains two primary alcohols, geraniol and
citronellol, both of which could be chlorinated quite easily but then may be difficult to completely separate from the rest of the oil. And OTC
essential oils are very expensive and probably best left as a posh person’s belief system…
Quote: Originally posted by Eclectic  | OK. Has anyone here ever heard anything about Barbier-Gringnard reactions? Alkyl halide, zinc dust, water as solvent? It's not exactly rocket
science....
Also kind of a hot topic for "Green" chemistry.
|
Ecclectic, so do you believe the ‘one pot reaction’ (as it’s described in Wiki):
Octyl chloride + acetone + Zn powder (all in water) === > 2-methyl-2-decanol + Zn(OH)Cl
… would effectively proceed? Octyl choride is of course not miscible with water to begin with… I somehow doubt that.
[Edited on 4-1-2011 by blogfast25]
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Quote: Originally posted by Eclectic  | OK. Has anyone here ever heard anything about Barbier-Grignard reactions? Alkyl halide, zinc dust, water as solvent? It's not exactly rocket
science....
Also kind of a hot topic for "Green" chemistry.
|
It should be noted that the Barbier-Grignard reaction is specific for propargyl or allyl halides that are highly activated.
Plain vanilla halides like propyl bromide will not work as far as I know.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
How do the reactivities of Grignard reagents compare for Cl:Br:I (all other things being equal)? I see a lot of references to bromides being used,
rather than chlorides. Sigma offer 1-bromooctane for £19.20/100 g.
Edit: reactivity seems to go I > Br > Cl
And what are good procedures to sufficiently dry the reagents?
[Edited on 4-1-2011 by blogfast25]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Bromide grignards are easier to start and react more smoothly than the chlorides.
Also, the alkyl bromides are very easy to make from the alcohol, KBr and H2SO4. 1-Butanol is a cheap precursor.
The chlorides are less easy to make, as HCl doesn't readily react with primary alcohols unless a catalyst like anhydrous ZnCl2 is present.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I see.
Sigma’s offer of £6.60 / 100 gram (0.606 mol) of 1-bromohexane is attractive. At assumed conversion rate of 90 % with acetone it would make about
80 g of 2-methyl-2-octanol.
From that same source, 1-bromobutane seems expensive: £5.70 for 25 gram, almost quadruple of the 1-bromohexane! Of course you can brominate 1-butanol
but HBr isn’t cheap either…
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Of course you can brominate 1-butanol but HBr isn’t cheap either… |
Hence why you use KBr and H2SO4. An excess of H2SO4 aids the reaction. NaBr will also substitute.
|
|
|
NurdRage
Hazard to Others
  
Posts: 182
Registered: 11-11-2010
Member Is Offline
Mood: No Mood
|
|
I was thinking, before we go off and start finding ways to make tertiary alcohols, it might be more prudent that we verify a particular long-chain
alcohol works before we try to make it.
Alfa Aesar has a huge array of alcohols for purchase, a fair number are tertiary.
Just to name a few:
4-methyl-4-nonanol (C10)
2,6-Dimethyl-2-heptanol (C9)
3-ethyl-3-hexanol (C8)
While they are not cheap, if one of them works then we know we're in the right area and can refocus our attention on making that particular alcohol or
its isomer. I think this would be a more efficient approach overall because It would be rather wasteful if we spent so much time and money trying to
make an alcohol that turns out not to work.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DJF90  | Hence why you use KBr and H2SO4. An excess of H2SO4 aids the reaction. NaBr will also substitute.
|
Yes but the REAL cost is the Br, in whatever form you use it… Using NaBr/KBr + conc. H2SO4 isn't going to be substantially cheaper. Not to mention
yield and work up concerns, as opposed to simply buying the n-alkyl bromide. I challenge anyone to match SA's n-hexyl bromide with a homebrew product
in total cost.
Quote: Originally posted by NurdRage  | I was thinking, before we go off and start finding ways to make tertiary alcohols, it might be more prudent that we verify a particular long-chain
alcohol works before we try to make it.
Alfa Aesar has a huge array of alcohols for purchase, a fair number are tertiary.
Just to name a few:
4-methyl-4-nonanol (C10)
2,6-Dimethyl-2-heptanol (C9)
3-ethyl-3-hexanol (C8)
While they are not cheap, if one of them works then we know we're in the right area and can refocus our attention on making that particular alcohol or
its isomer. I think this would be a more efficient approach overall because It would be rather wasteful if we spent so much time and money trying to
make an alcohol that turns out not to work. |
Broadly speaking agreed: it would be worthwhile checking whether 2-methyl-2-ol functionality is the only thing that works or whether tertiary 3-ols or
4-ols do the magic too… I’m gonna have a look at these products from Alfa Aesar, Nurdrage.
Edit:
That 2,6-Dimethyl-2-heptanol, for instance:
http://www.alfa.com/en/GP100W.pgm?DSSTK=B25691&rnd=72439...
£14.50 / 50 gram (BP 180C) is a bit of a steal, IYAM! The others you mentioned seem a little outside of my league on price...
[Edited on 5-1-2011 by blogfast25]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Tetrahydromyrcenol (2,6-dimethyloctan-2-ol, 18479-57-7) seems to be the cheapest saturated long chain t-alcohol available via chemical vendors. Si**a
sells it for 54 EUR/kg which is in the price range of solvents. All the other long chain t-alcohols are quite expensive, 2-methylhexan-2-ol included.
NaBr, KBr or NH4Br can be used for the bromination of lower primary and secondary alcohols. I never applied this method on n-butanol, but on isobutyl
alcohol (Me2CHCH2OH) it gave me a 33% yield of isobutyl bromide. However, I'm sure the reaction can be easily optimized to give yields above 60% on isobutanol,
n-butanol and possibly also on amyl alcohols and other alcohols immiscible in aqueous H2SO4. Otherwise, butyl bromide is very cheap, but making it is
fun and of good pedagogic value. Like I said, this method is not very suitable for higher alcohols, but these can be made into bromides by refluxing
them with 48% HBr(aq). This way I made 3-phenylpropyl bromide in good yields from 3-phenylpropan-1-ol. The reaction is extremely slow at room
temperature (only traces of bromide form), but only takes a couple of hours at reflux with good stirring (biphasic mixture).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@Nicodem: Excellent find on the tetrahydro myrcenol (2,6-dimethyloctan-2-ol, 18479-57-7)! SA UK = £38 / kg, £ 15 for a(n
unspecified) sample. BP ≈ 200C. This is probably a derivative from a natural product, hence the lower price and the trivial name.
@All: I’ve rearranged the proposed reaction mechanism a little bit, to try and focus the mind, here (the K thread):
http://www.sciencemadness.org/talk/viewthread.php?tid=14970&...
The four crucial reactions are:
2 KOH(s) + 2 ROH(sol) < == > 2 KOR(sol) + 2 H2O(sol) (I)
2 KOR(sol) + Mg(s) < == > 2 K(l) + Mg(OR)2(sol) (II)
Mg(OR)2(sol) + H2O(sol) < == > MgO(s) + 2 ROH(sol) (III)
K(l) + 2 ROH(sol) < == > 2 KOR(sol) + H2(g) (IV)
The third one is almost certainly the thermodynamic driver but probably not the rate determining step (RDS). Probably either the K alkoxide formation
or the K alkoxide reduction is the RDS.
Assuming I’m seeing this right, from there it might be possible to predict a ‘winning alcohol’. Which kind of boils down to some relatively
simple choices [cough!]:
1. tertiary alcohol function in 2 position or other position?
2. methyl, ethyl or other functions on the tertiary C-OH?
Personally I would have thought that longer R groups in a tertiary alcohol R-OH would render the -OH group more active because the R group is electron
rich, increasing the partial charge δ- on the electrophile oxygen atom. So that would favour ethyl functionality over methyl functionality. By
this reasoning 3-ethyl alka-3-ols would be more ‘active’ than 2-methyl alkan-2-ols, at least with respect to reactions (I) and (IV)…
[Edited on 6-1-2011 by blogfast25]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Yesterday and today I made a batch of 1-bromobutane.
I used:
50g 1-butanol
70ml conc. H2SO4
40ml water
100g KBr
Butanol and H2SO4 were mixed, then the water added (strong exotherm) and the mix poured upon the powdered KBr.
It was refluxed for about an hour and then slowly distilled over a free flame over the course of 2 hours.
The lower layer of the biphasic distillate was separated, washed twice with 10ml conc. H2SO4, then with water, Na2CO3 solution and water again, shaken
with CaCl2 until clear, and distilled over phosphorus pentoxide.
It came over completely at 100-101°C.
I obtained 79g, an astoundingly high 85% yield! I never had that kind of yield with the ethyl bromide prep.
In this 1-bromobutane prep, butanol is the limiting reagent and KBr is present in slight excess, while in the EtBr prep I generally use an excess of
ethanol.
The 1-bromobutane thus prepared has a slightly pungent smell component and fumes a slight bit in air. I wonder how this comes. I know this phenomenon
from purified chloroform that's been distilled over P2O5- it also fumes slightly, which I have no plausible explanation for.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Hey, that’s fantastic GC, great yield too! Hopefully the fuming won’t interfere with the Grignard reaction the 1-bromobutane is intended for.
|
|
|
| Pages:
1
2
3
4
..
19 |