| Pages:
1
2 |
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Preparation of Acetic Anhydride
Preparation of Acetic Anhydride
by Magpie
12/9/10
Introduction
This procedure is for the preparation of ~ 10 mL of acetic anhydride (Ac2O) using sulfur, bromine, and anhydrous sodium acetate (NaOAc). It is based
on the 4/29/09 post of benzylchloride1 of the ScienceMadness forum.
Preliminarily, disulfur dibromide (S2 Br2) is formed:
2S + Br2 --> S-Br-Br-S
Then, Ac2O is formed as follows, according to Thorpe (ref 1):
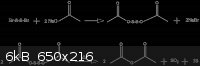
Orshansky and Bograchov (ref 2) indicate instead the following reaction:
8CH3OONa + S +3Br2 = 4(CH3O)2O + 6NaBr + Na2SO4
The reactions of Thorpe, however, are supported by the observations of this author of free sulfur and a liberated gas.
CAUTION
S2 Br2, a red, fuming liquid, is produced during this preparation. It has a terrible sulfur stench and is toxic. SO2 and hot Ac2O vapors are also
noxious. Therefore, this procedure must be conducted outside or in an efficient fume hood.
Full safety gear is recommended, ie: eye goggles, thick rubber gloves, long-sleeved shirt, and a lab apron.
Procedure
A. Equipment Set-Up
Secure an angled 3-neck 500 mL round bottomed flask (RBF) to a ringstand. Attach a pressure-equalizing addition funnel to a side-neck. Install a
reflux column equipped with a CaCl2 tube in the other side-neck. If available, install a mechanical stirrer in the center neck. If not, install a
plug. All glassware is to have ground-glass fittings, coated with a thin film of silicone grease, and clamped.
Provide cooling water for the reflux column.
B. Reagent Preparation
Weigh out 66.5g (0.81 moles) of anhydrous sodium acetate and set aside in a covered beaker. Weigh out 3.5g (0.11 moles) of sulfur in a beaker and set
aside. Have available 20 mL (0.39 moles) of dried Br2. (Note 1)
Note that bromine is the limiting reagent. The sulfur, except that released as SO2, is recycled in situ.
C. Ac2O Generation
Temporarily remove the center plug of the 500 mL RBF and pour in the anhydrous sodium acetate using a powder funnel. Reinstall the plug. Turn on the
cooling water.
Pour the 20 mL of dried bromine into a 100 mL beaker. Slowly mix in the sulfur with the bromine using a stirring rod. When the resultant S2 Br2 +
Br2 is homogeneous, pour it into the pressure-equalizing addition funnel (valve closed!) and install the plug in the fill port.
Slowly dribble in the S2 Br2 + Br2 onto the sodium acetate powder while stirring continuously mechanically, or intermittently by use of a stirring rod
inserted though the center neck. Replace the plug after any manual stirring. Achieving a homogeneous mix may be somewhat difficult. (See
Discussion below.) Keep stirring after all of the S2 Br2 has been added. If necessary, remove the pressure-equalizing funnel and
manually stir via the side port. Close the port with a plug when not in use. When the reaction finally takes off the products will turn into a
creamy slurry. Considerable heat will have been generated, bringing the slurry temperature as high as 80°C. Continue to stir for about 20 minutes.
The light yellow color of the slurry is due to the presence of the elemental sulfur byproduct and indicates that the Ac2O formation is nearing
completion. Continue stirring as the slurry cools to room temperature.
D. Vacuum Distillation
Set up for vacuum distillation of the Ac2O using the 3-neck 500mL RBF. Place an insulating blanket over the RBF. With a vacuum of 23”Hg (absolute
pressure = 178 mmHg) the Ac2O comes over at about 72°C. Yield of the crude Ac2O will be ~30 mL.
E. Simple Distillation
Set up for simple distillation of the crude Ac2O at atmospheric pressure. Use a 50 mL RBF as pot. Collect 2 cuts. The first cut (~15 mL) will come
over at about 128-135°C. The 2nd cut (~10 mL) will come over at 135-143°C. Literature value for the bp of Ac2O is 140.0 °C. Yield, based on the
10mL of cut 2 and the amount of Br2 charged, is ~27%.
Cleanup
Cleanup of the 500mL RBF should be straightforward with very little strong sulfur smell. There will be NaBr crystals and some minor char in the bottom
of the flask. This residue will wash out easily with hot soapy water.
Confirmation of Identity
There are several qualitative tests that can be used for the confirmation of acid anhydrides (Note 2). One that is particularly dramatic is the
formation of acetanilide upon mixing of Ac2O with aniline. The acetanilide can then be re-crystalized and a melting point determined (113-114°C).
Discussion
The reactants can initially be difficult to mix following the addition of the S2Br2 to the dry NaOAc. This appears to be why some variants of this
method, with either Br2 or Cl2, also use additional amounts of the anhydride product to thin the mix of reactants. Do it on a small scale, then take
the crude anhydride and use it as a 'solvent' for a larger run.
Notes
1. The use of anhydrous reagents is important. S2 Br2 decomposes in water. If the dryness of the NaOAc is in doubt it should be fused per the
directions found in Vogel’s Practical Organic Chemistry, 3rd Ed, Section II, 50, 9, p. 197.
2. See: http://www.chemistry.ccsu.edu/glagovich/teaching/316/index.h...
References
1. Thorpe’s Dictionary of Applied Chemistry, vol. 1, Revised Edition, 1921, p 28. (Thanks to Polverone for this interesting and obscure
information.)
2. “Laboratory Method for the Preparation of Organic Acid Anhydrides,” by Jehuda Orshansky and Eilahu Bograchov, Chemistry & Industry, 44,
November 4, 1944, p. 382. (Thanks to Sauron for pointing this out in the Rhodium archives.)
3. Thanks to not_important for the tip about adding preliminary product anhydride to the NaOAc as solvent to facilitate reactant mixing.
[Edited on 9-12-2010 by Magpie]
[Edited on 11-12-2010 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Good work! So this method is confirmed as working, though with relatively low yield.
The sulfur dissolves rapidly in the bromine, doesn't it? I tried this out myself a long time ago, but was discouraged by the very broad boiling range
of the crude product.
Are you planning to try out the Na acetate + SO2 + Cl2 method as well?
Did you make the anhydrous sodium acetate yourself? If yes, tell me more.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by garage chemist  | Good work! So this method is confirmed as working, though with relatively low yield.
The sulfur dissolves rapidly in the bromine, doesn't it? I tried this out myself a long time ago, but was discouraged by the very broad boiling range
of the crude product.
Are you planning to try out the Na acetate + SO2 + Cl2 method as well?
Did you make the anhydrous sodium acetate yourself? If yes, tell me more. |
Yes, this method has been confirmed by me several times and I believe by benzylchloride1 also.
Yes, the S2Br2 formation is rapid and without challenge.
I have no plans to try out any other method as I was only interested in getting small amounts for other experiments.
I bought the anhydrous NaOAc off eBay. But I understand entropy51 has a facile method for making his own.
The real challenge is just getting everything to continue mixing after the S2Br2 is added to the NaOAc. I have a mechanical mixer with ptfe bearing,
shaft, and prop. But I still have to dig in with glass stirring rods from the side ports. Once the reaction takes off and the mix goes creamy
there's no more problems.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
The mixing problem appears to be why some variants of this, with either Br2 or Cl2, also use additional amounts of the target product to thin the mix
of reactants. Do it on a small scale, then take the crude anhydride and use it as a 'solvent' for a larger run.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by not_important  | The mixing problem appears to be why some variants of this, with either Br2 or Cl2, also use additional amounts of the target product to thin the mix
of reactants. Do it on a small scale, then take the crude anhydride and use it as a 'solvent' for a larger run.
|
I agree, although I have not yet tried this. I will add this note to the procedure.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Congratulations on your succes! This is a very useful reagent! It's of no use for me because I have plenty of Ac2O, but I still like to see that it
worked.
What is the first fraction of 15mL that came over?
I asume this is also acetic anhydride, maybe some azeotrope?
Test this material by adding a mL to a mL of water. It should not mix and form two layers, but react when very quickly when heated to boiling. Or do
the test by reacting it with aniline.
It also might be acetic acid. What kind of sodium acetate did you buy? From a supplier selling plastic bags of the stuff for use in the experiment
'Hot Ice'? Maybe he mentioned it as anhydrous, but it might be partially or fully hydrated, and not truly anhydrous as the anhydrous material is not
needed for the Hot Ice experiment and is more expensive, so it might be a typo on eBay. Or is it a reagent grade material?
You can maybe test how much water it contains by putting a weighed amound in the oven, and weighing it again after it has fully dehydrated in the
oven.
[Edited on 20-12-2010 by Jor]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thank you Jor. No, I have not done any research on the nature or usefulness of the discarded fraction. I was only interested in getting small
amounts of Ac2O for some planned experiments.
The NaOAc was, as you say, obtained off eBay in a plastic bag. The seller claimed it to be anhydrous. It is very white and free-flowing. I can't
remember if it was advertised as "hot ice" material. Much of it is.
[Edited on 20-12-2010 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
Small note for anyone who needs dehydrated NaAc.
Any remaining water can be removed using microwaves.
The material doesnt heat up when there is no water left.
What a fine day for chemistry this is.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Good idea; sure beats fusing the stuff.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by User  | Small note for anyone who needs dehydrated NaAc.
Any remaining water can be removed using microwaves.
The material doesnt heat up when there is no water left.
|
NaOAc. Very different than NaAc (Which I'm not even sure would be a stable compound).
Are you sure that it ceases to heat? I can tell you from experience that CaCl2 and MgSO4 both do continue to heat. The MgSO4 got so hot that the glass
dish it was in melted on the bottom.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
Sorry for being unclear, I tested this with NaOAc (sodium acetate).
Yes I can confirm this from multiple tests, the material when dry did not heat whatsoever, tests where performed in a keramic dish.
Also no charring occured.
Specific runtimes/wattage I cannot remember for I dont have my notes nearby.
My guess would be that I fired it up in 30 to 60 sec cycles.
Be aware that when it is heated and still contains water superheating can occur.
Also it will start to bubble and thus grow in size dramatically.
Don't hessitate to ask in case I forgot anything.
What a fine day for chemistry this is.
|
|
|
tnphysics
Harmless

Posts: 18
Registered: 29-12-2010
Member Is Offline
Mood: No Mood
|
|
Can acetic acid be used instead? Then it might be easier to recover the bromine by electrolysis.
I LOVE science!
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Waffles SS recently made a comment about the the poor yield of this procedure, ie, ~27%.
Orshansky and Bograchov (see reference 2 of my procedure) reported a yield of 87.5%. However, their procedure was significantly different than mine
in 2 respects, ie:
1. Their scale was 6.7X that of mine.
2. They used 50g of acetic anhydride as solvent.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Did you try S2Cl2 method?
[Edited on 5-3-2011 by Waffles SS]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
No, for the same reason that Orshansky and Bograchov chose not to: bromine is easier to work with than chlorine.
[Edited on 5-3-2011 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
May you give us some pictures of this method?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
See: http://www.sciencemadness.org/talk/viewthread.php?tid=9096&a...
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
SM2
Hazard to Others
  
Posts: 359
Registered: 8-5-2012
Location: the Irish Springs
Member Is Offline
Mood: Affect
|
|
could a swap work here, using easily made propionic anhydride, and glacial aceetic?
|
|
|
tetrahedron
Hazard to Others
  
Posts: 210
Registered: 28-9-2012
Member Is Offline
Mood: No Mood
|
|
you'll end up with the mixed anhydride as a serious byproduct.
|
|
|
x3110n
Harmless

Posts: 2
Registered: 8-9-2017
Member Is Offline
Mood: No Mood
|
|
I know that you mean this in the most positive way, but superheating will NEVER occur when a substrate like sodium acetate is present in that ratio to
the water. The crystals privide more than enough porous surface to help the convertion in to steam at 100 degreess C.
Just saying
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by x3110n  |
I know that you mean this in the most positive way, but superheating will NEVER occur when a substrate like sodium acetate is present in that ratio to
the water. The crystals privide more than enough porous surface to help the convertion in to steam at 100 degreess C.
Just saying |
ORLY?
As some one who's don this more then once, and cleaned the microwave more then once super heating and mini steam explosions most certainly happen if
you don't stop and stir the crystals.
May I ask what procedure you used to avoid this?
|
|
|
Feriman
Harmless

Posts: 6
Registered: 15-3-2019
Member Is Offline
Mood: Learning
|
|
what is The most accurate way to test acetic anhydride?
how can I seperate acetic acid and purify acetic anhydride?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Make a derivative (as I explained above) and determine its mp.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The separation of Ac2O from AcOH is the harder question. I can't think of anything but fractional distillation (bps 119 vs 140).
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Magpie,
I want to suggest GB299342 patent for making Ac2O.I think you made HPO3 from Phosphoric acid before.
[Edited on 19-4-2019 by Waffles SS]
Chemistry = Chem + is + Try
|
|
|
| Pages:
1
2 |