| Pages:
1
..
3
4
5
6
7
..
30 |
arsphenamine
Hazard to Others
  
Posts: 236
Registered: 12-8-2010
Location: I smell horses, Maryland, USA
Member Is Offline
Mood: No Mood
|
|
n.b. the c=c bond is c2-c3
The trivial name for the aliphatic homolog is 2-methyl 3-phenyl propionaldehyde.
|
|
|
Pope
Harmless

Posts: 10
Registered: 14-10-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sedit  | Quote: Originally posted by Pope  | How would one approach trying to separate an azeotropic mixture of Toluene/Methyl Ethyl Ketone, my goal is to completely separate and purify the
existing Toluene.
The components exist as - 430g Toluene: 410g MEK.
Thank you for your assistance, I have tried the search engine with little success. |
Sodium Metabisulfite will form a complex precipitating the MEK as an adduct. Repeated washes of the MeBn with Bisulfite solution should clear up your
problem. |
In order to run this reaction wouldn't you use ethanol to dilute the solution in turn forming another azeotrope with the toluene?
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
I have few questions:
1. I have nail polish remover that has MEK, water, isopropyl alcohol, methoxyisopropanol, calcium chloride. I want to precipitate MEK with potassium
metabisulfite. After that, how can I extract MEK from that extract without distillation?
2. Can I stabilize chloroform with brandy?
3. Is there some use for Copper (II) acetate in organic chemistry?
4. How could I extract chromium from stainless steel?
Thanks for answers 
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
1) No idea.
2) Should work, add brandy, some ethanol will go in to the chloroform phase, then separate the aqueous phase. But your chloroform will be wet and also
likely contaminated with miscellaneous crap from the brandy that prefers to be in a non-polar solvent; why not distill the brandy first to get
something closer to pure ethanol?
3) Yes
4) There is a thread on this here somewhere... see http://www.sciencemadness.org/talk/viewthread.php?tid=13347#...
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
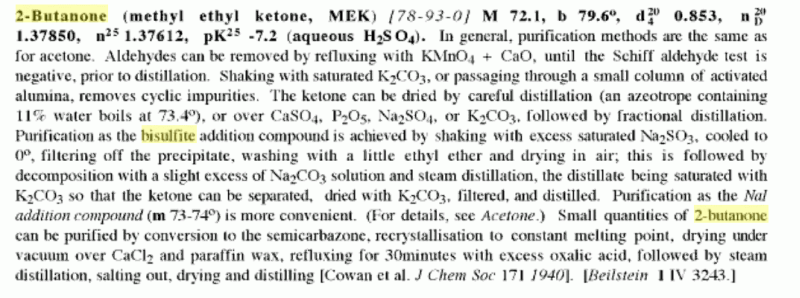
The bisulfite-MEK adduct is fairly water soluble, even with a saturated solution of bisulfite some of the adduct remains in solution. Given the CaCl2
in it, best to first distill and collect the portion boiling at or below 80 C, then make the addition product.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Quote: Originally posted by Pope  |
In order to run this reaction wouldn't you use ethanol to dilute the solution in turn forming another azeotrope with the toluene?
|
No need for ethanol since you don't wish to precipitate the adduct just wash it with Bisulfite solution.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Pope
Harmless

Posts: 10
Registered: 14-10-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sedit  | Quote: Originally posted by Pope  |
In order to run this reaction wouldn't you use ethanol to dilute the solution in turn forming another azeotrope with the toluene?
|
No need for ethanol since you don't wish to precipitate the adduct just wash it with Bisulfite solution. |
And the resulting adduct will dissolve in the bisulfite solution?
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Does anyone know if AcOH would react with CaCl2?
Im pretty sure it doesn't but before I use it as a drying agent to report the yeilds on my Dichloromethane extraction of acetic acid I want to be 100%
sure it won't give me issues. I would normally use MgSO4 for this but I am all out and forgot to pick some up.
Acetic acid was made from Sodium acetate and Sodium Bisulfite and the addition of DCM showed a small H2O layer on the top that I want to do away with
before filtering since it will hold more AcOH then I want it to decreasing yeilds.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Most CaCl2 contains some Ca(OH)2 and/or CaCO3, which obviously don't go with AcOH. Also CaCl2 forms adducts with compounds containing -OH, -NR2
(especially -NH2), and C=O; while not strictly a reaction and of less strength than the CaCL2-H2O adduct, they will get pulled from solutions to some
extent. Strong aqueous CaCll2 solutions will be less absorbent of those compounds, still Na2SO4 or MgSO4 would be a better choice.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
The desired goal is to use anhydrous CaCl2 and add just a small amount to dry DCM/GAA mixture. Without very much H2O present (and non when its done
its job)do you feel it will still have this problem?
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
DeAdFX
Hazard to Others
  
Posts: 339
Registered: 1-7-2005
Location: Brothel
Member Is Offline
Mood: @%&$ing hardcore baby
|
|
I did some research on protecting graphite anodes in a chlorate cell. Coating the anode with polystyrene, linseed oil or some other material will
result in a more durable anode.
I decided to coat my anode in polystyrene. After doing so my anode is bubbling much less in comparison. Is less bubbling indicative of a more
durable anode? Did I apply too much and has my efficiency gone down?
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Seems like you will want to measure current flow to have an idea of what is going on. It seems *likely* that you coated it too thickly and that the
polystyrene has formed an electrically resistant layer, but measuring the amps would give you a clearer picture of what's going on.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
I tryed extracting AcOH using acetone but I fear excess Sulfuric acid left over in the Acetic acid synthesis has led to an aldol condensation product
of somekind.
It smells like cat piss or some other foul smell and appears to be dark brown in color but this could be impurities in the sulfuric acid used.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|

Uploaded with ImageShack.us
...trying to name this compound....some help....solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
3-phenyl-2-methyl-propanoal (or propionaldehyde) oxamine is what I would call it, at first look. Likely someone who keeps up on terminology will
correct that, so I get to learn something.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Quote: Originally posted by not_important  | 3-phenyl-2-methyl-propanoal (or propionaldehyde) oxamine is what I would call it, at first look. Likely someone who keeps up on terminology will
correct that, so I get to learn something.
|
....thanks, you gave a good educated name, here is the name of the material.......2-methyl-3-phenylpropanal it's not the oxime but that will be easier
to find......with the main skeleton name

Uploaded with ImageShack.us
......source,
http://www.chemsynthesis.com/base/chemical-structure-6195.ht...
[Edited on 28-10-2010 by solo]
[Edited on 28-10-2010 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Yeah, forgot the rule that numbers goes from low to high, payed too much attention to the mass of the radical. Duh ... Old age is taking its toll.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
....has there been any reports of recimization when doing reductions of halogens with compounds with chiral centers , case and point and iodated
phenylalaninol HCl.....based on a reading with a refractometer the index points to a recemic amine......solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
what i would get if i would mix poor man's aqua regia (hcl + nh4no3) and benzoic acid? COuld I get some useful product out of this and I read
somewhere that aromatic compounds give chloropicrin with aqua regia, is this true?
I would also like to make p-aminobenoic acid without nitration of benzoic acid (i don't have any sulfuric acid). Is there another route to it from
benzoic acid?
[Edited on 4-11-2010 by Random]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Benzoic acid is a bad starting point for p- and o- substitution, meaning it's just about worthless for making PABA. There is a recent thread on
making PABA, within the last week or so; read it and then ask further.
|
|
|
Bolt
Hazard to Others
  
Posts: 188
Registered: 26-3-2007
Member Is Offline
Mood: No Mood
|
|
Hi, I'm wondering whether anyone can point me toward a good review or book chapter describing elemental calcium as a reducing regent in organic
synthesis.
|
|
|
DeAdFX
Hazard to Others
  
Posts: 339
Registered: 1-7-2005
Location: Brothel
Member Is Offline
Mood: @%&$ing hardcore baby
|
|
I plan on making some copper nitrate via double displacement of a nitrate salt and copper sulfate.
Which nitrate salt will produce the easiest to filter sulfate percipitate? Strontium or Barium?
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
I have Nickel acetate and need Nickel chloride....is there a way to convert it to the chloride salt ....have looked in google and found
nothing....will most likely be found in some inorganic text...will keep looking, but if someone knows i would appreciate the assist.....solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
I would think roasting the acetate would leave you with the oxide which HCl would convert to the chloride but this is just an assumption with little
thought put into it to be honest.
I have a question. I got a paint thinner that contains acetone,DCM,MeOH,Toluene and KOH.
I can not seem to seperate the layers very well using water and distillation bumps so bad even with boiling chips. Any suggestions on whats going on
here. Im shocked DCM and KOH can even be in the same solution since my instincts tell me it would be a bad idea but I guess it can.
I only want the toluene and DCM so perhaps steaming it would be my best option I guess. Right now its my cheepest over the counter source of Toluene.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
arsphenamine
Hazard to Others
  
Posts: 236
Registered: 12-8-2010
Location: I smell horses, Maryland, USA
Member Is Offline
Mood: No Mood
|
|
N-(2-methyl-3-phenylpropylidene)hydroxylamine
To search PubChem or ChemSpider, try the SMILES string first.
The InChI string may be overkill. I'm guessing that stereochemistry isn't too important here.
SMILES: CC(CC1=CC=CC=C1)C=NO
InChI=1S/C10H13NO/c1-9(8-11-12)7-10-5-3-2-4-6-10/h2-6,8-9,12H,7H2,1H3
ACD/Labs has a freeware name generator that is limited to 50 atoms per compound including H. The commercial lite version is ~$150 and is
quite good. IUPAC, the nomenclature org, gives it their blessing.
ChemAxon's MarvinSketch is almost freeware, has an academic license hook, but handles larger structures. Not as slick as ACD/Name, but v. useful in other
ways as well.
[Edited on 9-11-2010 by arsphenamine]
|
|
|
| Pages:
1
..
3
4
5
6
7
..
30 |