Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Butyric acid from potatoes and water
I want to produce butyric acid with potatoes:
http://www.youtube.com/watch?v=18K1wQv59h8
Now when I want to remove butyric acid from the mix, I need information for solubility of butyrate salts. Which metal carbonate should be the best for
isolating butyric acid? It would be good if it was unsoluble while acetate would be soluble so I could seperate these two.
Is there any other way of isolating butyric acid from that?
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
What a dumb video, rotten potatoes is not butyric acid.
If you wanted to you could make 'soap' out of butter and NaOH, remove the glycerin and add HCl to get your acid.
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
stygian
Hazard to Others
  
Posts: 242
Registered: 19-9-2004
Member Is Offline
Mood: No Mood
|
|
*Rancid butter
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Quote: Originally posted by mr.crow  | What a dumb video, rotten potatoes is not butyric acid.
If you wanted to you could make 'soap' out of butter and NaOH, remove the glycerin and add HCl to get your acid. |
but then I would get stearic acid
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Different oils are made from different fatty acids. Butter happens to have lots of butyric, hence the name 
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
The butyric acid is the most noticable free acid in rancid butter due to its smell.
But it is not a major component;
http://www.webexhibits.org/butter/compounds-fatty.html
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
I also looked at butyric acid from butter and saw that it was impractical - just a few % of the total fatty acids.
The video is kind of retarded, time that could have been spent explaining what is going on is instead devoted to video of a potato and someone slicing
it. Yeah, I needed that demonstrated.
That said of course it's possible to produce butyric acid by fermentation. Google should provide some information on how to do this, though if I were
just going to wing it I'd try potatoes, water, and some raw milk swiss cheese as a culture (all under anaerobic conditions).
I suspect that butyric acid is already long-chain enough that the best way to separate it would be by salting the free acid out of the aqueous phase,
rather than by trying to form an insoluble salt. Of course you'd need a further workup to separate the acid from other non-polar crap but fractional
distillation should be possible.
|
|
|
HydroCarbon
Hazard to Self
 
Posts: 77
Registered: 7-7-2008
Location: Anytown, USA
Member Is Offline
Mood: No Mood
|
|
Assuming you can get butyrate; butyric acid, having a relatively short alkyl tail should be soluble in water. Therefore precipitating it out as a salt
would probably be your best bet.
Precipitate your organic salts out of the raw source, acidify, then distill. You might need to extract with solvent after acidifying if you use HCl
to avoid distilling over HCl.
I'm not exactly sure what salts will precipitate out of aqueous solution, but I'd be willing to bet that calcium would.
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
You could probably just add a stronger acid to it, such as HCl, than saturate it with salt. If there is enough to even bother with the butryic acid
could probably be extracted into ethyl acetate and than later separated from that.
|
|
|
spong
Hazard to Others
  
Posts: 128
Registered: 28-5-2009
Location: Chatham
Member Is Offline
Mood: No Mood
|
|
I've been wanting butyric acid for years, the esters just smell so good! The calcium salt is insoluble and you'll need a solvent to get the acid from
water, anything above propionic isn't that soluble in water and could be extracted with something less polar. If anyone has any success in getting it
from butter it would be great but distilling butyric acid is not something I'd like to do at home. I was thinking about skipping the acid and making
ethyl butyrate via the malonic acid ester synthesis if I could make malonic acid easily.
-After reading up on the potato method it might not be that far fetched, I saw a method that used potatoes, swamp water, dirt and spit. The spit would
have amylase in it to break down the starches into sugars, and the dirt and water could perhaps have C. butyricum to make butyric acid from the
sugars? The oil could be there to stop oxygen getting in as C. butyricum is anerobic, the video said the jar without the oil didn't smell. The swamp
water could also be for the anerobic bacteria, stagnant and oxygen depleted.
Either way I'll give it a try, I have a plenty of jars, potatoes, dirt and spit 
[Edited on 28-9-2010 by spong]
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
According to the old books, butyric acid can be prepared by fermentation of potatoes or starch, but the prep is not quite as simple as that. CaCO3 is
added to buffer the pH and trap the acids as Ca salts. Rancid cheese and spoiled milk are usually specified as the starter culture. I've never tried
this.
Butyric acid was orginally isolated from butter (which need not be rancid) by saponifying with K2CO3, dissolving the soap in ethanol and distilling
with an excess of sulfuric acid. This can easily yield enough butyric acid to synthesize a little pineapple ester, enough to easily smell.
|
|
|
rrkss
Hazard to Others
  
Posts: 193
Registered: 18-12-2009
Member Is Offline
Mood: No Mood
|
|
If you can get your hands on 1-propanol. You can always react it with NaBr/H2SO4 to from 1 propyl bromide. Then react that with magnesium in ethyl
ether to form the grignard and react that with CO2 which is very easy to get (heck even degassing soda water will do the trick) to form butyric acid.
The reaction needs to be anhydrous so you can use soda water to generate the CO2 and connect the gas generator to a CaCl2 drying tube to remove water
vapor.
[Edited on 9-28-10 by rrkss]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
I'll try to dig up the 19th century prep for lactic and butyric acids. They use sugar, potatoes supply starch that bacteria hydrolyse to sugars;
starting with mainly refined sugar should give a cleaner product.
Isolation is best started by acidifying and distilling the acid+water off, adding more water or passing steam through if needed to keep the distilling
flask filled with enough liquid. This will leave behind unconsumed starch and sugars, bacteria carcasses, and so on, as well as much of the
unconverted lactic acid. You'll get a mix of butyric, propanoic, acetic, and a small amount of higher acids, various steps can be used to isolate
them.
|
|
|
spong
Hazard to Others
  
Posts: 128
Registered: 28-5-2009
Location: Chatham
Member Is Offline
Mood: No Mood
|
|
So far my jar has some little bubbles appearing, CO2 hopefully, no stench yet though. I'm planning on filtering, adding CaOH, filtering, keeping the
precipitate then acidifying and distilling it off. It will definitely have some other acids in it but if it works I can try it with sugar and old milk
on a larger scale... once I have a fume hood.
Edit- I went to check it after I got home from work and found that is had been producing quite a bit of CO2, enough to make all the potato chunks
float to the top and foam out of the sealed jar. The liquid ended up on my desk, luckily it doesn't smell too bad yet, I can definitely detect a tiny
bit of butyric in it though. I've left some milk out to have a try with sugar+sour milk, I might use some of my winemaking equipment to try fermenting
a demijohn full of it 
[Edited on 1-10-2010 by spong]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Remembered to dig it out:
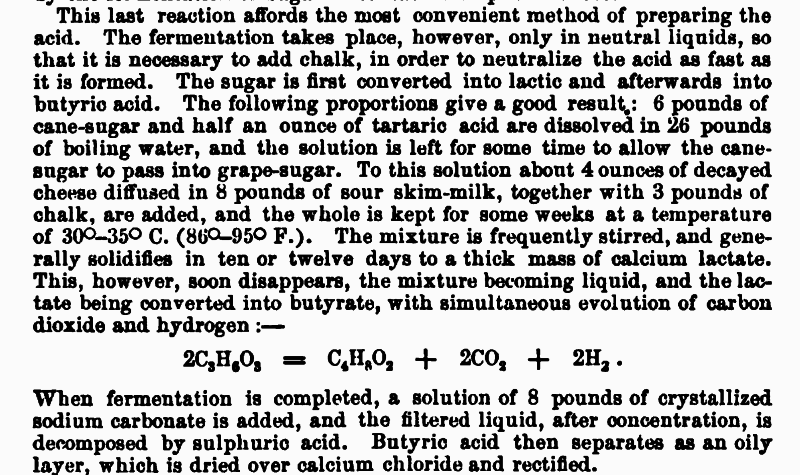
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Thanks a lot for the preparation method not_important, I will try it when I will have time. It's very good that I don't even need to distill it, it
will separate as an oily layer. But doesn't this yield calcium butyrate and whats the purpose of sodium carbonate? If the acid is in butyrate salts,
how is then separated into the pure form without adding the stronger acid?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Random  | | It's very good that I don't even need to distill it, it will separate as an oily layer. But doesn't this yield calcium butyrate and whats the purpose
of sodium carbonate? If the acid is in butyrate salts, how is then separated into the pure form without adding the stronger acid?
|
Not only you need to distil it, you need to "rectify" it. If you do not know what rectify means, it is essentially the same as distillation trough a
distillation column. The sodium carbonate is added to keep the medium basic and prevent the evaporation of butyric acid (now kept as butyrate) during
the concentration of the filtrate. After the concentration the butyrate is converted to butyric acid by acidification. All this is nicely described in
the last paragraph above. It is a fermentation based production, so a more or less complicated process of isolation and purification is unavoidable.
You can not just expect butyric acid will jump out of that nasty soup into a flask.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
As Nicodem said.
I'll add that the purpose of the sour milk is both to add desired bacteria and to provide protein, potatoes will provide protein and you could try
some experimentation using them plus a smaller of acidophilus milk that you let set at room temperature for a half day or so, or active culture
yogurt.
One method of improving the purity of the product is to do a determination of the relative amounts of acetic vs higher acids, and not fully acidify.
Acetic acid is a bit stronger than the higher homologues, leaving enough of the acid salt (sodium in this case) and distilling results in mostly
acetic acid staying as the salt while the free higher acids distill off. You still need fractionation (rectification), but it's a little easier.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
The sodium carbonate reacts with the calcium butyrate, undergoing double decomposition, and giving a solution of sodium butyrate and a precipitate of
calcium carbonate.
Ca(OOC-C3H7)2 + Na2CO3 ---> 2 NaOOC-C3H7 + CaCO3
That way you won't have CaSO4 precipitate in your boiling flask upon adding H2SO4. You really wouldn't want to boil a CaSO4 suspension (uncontrollable
bumping).
[Edited on 2-10-2010 by garage chemist]
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
I completely missed the last paragraph. Though, I can also do it with acidifying with HCl and then salting the butyric acid out. If I remember calcium
chloride salts the butyric acid as an oily layer so I can just use calcium butyrate solution and add hcl to make butyric acid and salt it out at the
same time. The product wouldn't be so pure but it would be useful for reactions.
|
|
|