FreeRadical
Harmless

Posts: 2
Registered: 23-8-2010
Member Is Offline
Mood: Perplexed
|
|
Hydroxylamine HCl synthesis question
Hi, firstly, this is my first post here so a big hello to everyone and I hope you'll be gentle if you think my question is dumb 
I'm attempting to prepare sodium hydroxylamine disulphonate as per Brauer page 528 (and ultimately hydroxylamine hydrochloride), only using 1mol of
sodium metabisulfite rather than 2mol of bisulfite - which should be the same I think.
NaNO2 + 2NaHSO3 = HON(SO3Na)2 + NaOH
69g of the nitrite was dissoved in 127ml distilled water and chilled.
190g og the metabisulfite was dissolved in 385ml water and also chilled.
(dilutions were taken from published solubilities)
The nitrite solution was added to 200g of chopped ice and the sulfite solution added slowly with stirring.
Everything seemed to go well, the reaction was still cold at the end, there was no gas evolved and the noxious odor of SO2 was virtually gone.
The procedure next calls for a saturated solution of 150g potassium chloride to be added before before being allowed to stand and the disulphonate to
crystalize out.
Can somebody help me understand what is happening when the KCl is added? Is there a reaction here or are we just salting out? Can NaCl be used
instead?
Any help would be very much appreciated, my chemistry knowledge seems to have developed many holes in the 20 years I've not applied it.
Cheers.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
I just woke up, and I have no idea how this reaction proceeds.
I'm imagining, that the problem here, is that you don't have any KCl handy.
In my current state of stupification, I not going to speculate, as to why the original experimenters chose KCl over NaCl.
The easiest thing to do, is to go out and buy some KCl, at least for this first run. Improvise later.
KCl is sold in many large US grocery stores, as a salt substitute. It usually resides in the condiments section. Nu-Salt is the name of the product
I use. About a dollar and a half, for three ounces ~85 grams. 99% KCl, less than 1% K Tartrate, SiO2, etc.
Three bucks. 170 Grams KCl. Hopefully pure enough for your purposes.
|
|
|
FreeRadical
Harmless

Posts: 2
Registered: 23-8-2010
Member Is Offline
Mood: Perplexed
|
|
You guessed my problem correctly, I had no KCl and, lacking in patience, decided to give it a go with salt instead with dissapointing results (no
crystals) - although maybe I just need to bang more in there. I expect the sodium hydroxylamine disulphonate is much more soluble than the potassium
salt.
I did consider using the low sodium salt substitute but the stuff here is only 60% KCl so didn't bother. I've got a load of nitrite and bisulfite
though so I'll see what happens when the KCl I ordered arrives in a couple of days.
I'd still like to know how this reaction works though so if anything comes to mind....
|
|
|
densest
Hazard to Others
  
Posts: 359
Registered: 1-10-2005
Location: in the lehr
Member Is Offline
Mood: slowly warming to strain point
|
|
The 100% KCl salt substitute is rarer, but might be available with searching. KCl may be separated from NaCl using the different solubilities in hot
and cold water. KCl 57:24 NaCl 39:36 Dissolve as much as possible in boiling water and cool. KCl will crystallize out first (to a large degree). You
should get 90% or better KCl.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
I Oregon, I buy my Nu-Salt KCl, at either Fred Meyers, or WinCo......Some Safeways carry a similar product.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
KCl is also available as a fertilizer, muriate of potash. I have some, but it is pretty gross. I would recrystalize it before using.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
if you can make do with the sulfate (which I think is easier to use as it does not
absorb water from the air)
then this might make things easier for you.
shroomedalice
Global Moderator
*****
Karma: +13/-2
Offline
Posts: 1023
View Profile WWW Email Personal Message (Offline)
Hydroxylamine Sulfate
« on: 28 June 2010, 14:45:31 »
Reply with quote Modify Remove Split Topic
hey guys been playing with a patent on making hydroxylamine sulfate made from sulfuric acid and nitromethane
neat.
well got it too work and it kills all cladenstine methods of making this substance.
in a 500ml flask was placed 125 ml of nitromethane.
this is refluxed and stirred.
when solution is refluxing 125ml of sulfuric acid is slowly (yes I mean fucking slowly) dripped into
the solution.
addition of sulfuric acid should take about 1 too 2 hours.
entire reaction should take 5 hours from time drip starts.
when 5 hours is compleated add mixture to equal weight alcohol and leave to cool.
filter out quantitive yeild of hydroxylamine from nitromethane.
this is the cheapest and quickest way known. it is also the most space efficient making
the whole small lab size idea more of a reality.
I was able to make over half a kilo in a day in a 500ml flask.
note of caution though this reaction releases carbon monoxide.
the condensor must be conected to a gas exaust of some kind that is feed
out side.
if you dont understand why carbon monoxide is dangerous for fucks sake do some reading.
other than that its a peice of cake.
e3500 console login: root
bash-2.05#
|
|
|
Hexagon
Harmless

Posts: 45
Registered: 11-5-2010
Member Is Offline
Mood: Fanf*ckingtastic
|
|
Have you tried adding CaCl2? Calcium salts use to be more insoluble than it's sodium or potassium counterparts, although being this all inorganic
stuff you might end up with a very soluble salt instead 
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
The reaction of nitromethane with aqueous mineral acid forms the hydroxylamine salt and formic acid, but I think this reaction may require heating,
please correct me if this is wrong.
| Quote: |
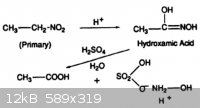
The action of strong aqueous acid on the primary nitroparaffins yields hydroxylamine as the acid salt, along with a carboxylic acid by-product (Fig.
8). The reaction proceeds through a hydroxamic acid intermediate.
"Speciality Chemicals: Innovations in Industrial Synthesis and Applications", edited by B. Pearson, p121 |
Unless I am mistaken, this reaction is one of the ones which bears the name of Victor Meyer, if you want to do more research into the subject (Victor
Meyer, Ann. (1876) p.663). The Nef Reaction (John Ulric Nef) is similar, using mineral acid to convert nitroethane into acetaldehyde and nitrous
oxide. Apparently, the reaction can go either way, depending on reaction conditions, though I am not sure what exactly these conditions are.
(obviously it does not matter whether it is nitromethane or nitroethane)
If someone else knows more about the details of this reaction and chemistry, please share.
Something else that should be noted, nitromethane can be used as a solvent for concentrated nitric acid, apparently without reaction. I have
seen nitromethane specified as the solvent in some nitration reactions using white fuming nitric acid. I am not exactly sure if the non-reactivity of
the nitromethane has to do with the reaction not being heated, or if the acid is so concentrated that no water is available to cause hydrolysis.
[Edited on 13-7-2013 by AndersHoveland]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
So nitromethane + HCl would make hydroxylamine HCl?
I'm looking for a method of using bisulfite and nitrite without SO2(3?)
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Yes, ATX made somthing about it in the prepublication. Glyoximates salts I beleive.
I never asked for this.
|
|
|
HoH
Harmless

Posts: 15
Registered: 15-11-2010
Member Is Offline
Mood: No Mood
|
|
What % sulfuric acid is used? If 125ml is used, is OTC sulfuric acid a viable source?
|
|
|
HoH
Harmless

Posts: 15
Registered: 15-11-2010
Member Is Offline
Mood: No Mood
|
|
Reflux Times.
Why are some people claiming this reaction can be completed in 5 hours, while others are claiming a 24 hour reflux is necessary? Obviously, you would
err on the side of caution and let the reaction go 24 hours, but I have noticed in the past that a lot of synths posted online are overly time
consuming. Was this 24 hours reflux determined based on periodic TLC readings?
Would it be more advantageous in terms of yield, to start the addition of HCL/Sulfuric acid after the nitromethane has achieved reflux?
Here is a good link re: large scale production of hydroxylamine. http://www.orgsyn.org/demo.aspx?prep=cv1p0318
[Edited on 4/5/2014 by HoH]
Spinnbarkeit in my flask.
|
|
|