| Pages:
1
2 |
Reference
Anders Hoveland

Posts: 13
Registered: 12-7-2010
Member Is Offline
Mood: No Mood
|
|
"Beware of Astrolite in cylindrical tubes of high shock impedance. It
loves a good LVD (low velocity detonation) under such conditions and
most anything is liable to set it off. The various Astrolite formulations are subject to LVD
when confined in material having high shock impedance.
This is a common property of many liquid explosives
and leads to extreme shock sensitivity under certain
conditions. BTW, Astrolites A and G were pussycats compared to some of the other
letters that nobody ever wrote about in public. "
8 Jul 1996 08:01:57 GMT, letter by Gerald L. Hurst (glhurst@onr.com)
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
What he meant by that was "don't mix that material & place it within a bottle". Maintain the "Sprengle" methodology when contemplating using
"Astrolite"; unless you have a container for fluids that is soft.....almost anything one can think of would have high shock impedance. The reason why
he used the term "pussycats" is that the material has been so mislabeled as the "most powerful-non-nuclear explosive"....
EDIT:
I spoke with Hurst at length during the mid-1990's & he had a serious antipathy toward crap-books that maintained that Astrolite was some type of
magic explosive. In fact, he believed that "dumbing down" the synthesis of explosives to a "recipe" was one of the most dangerous & ultimately
stupid things anyone could do. If people did not pay their dues in reading and studying, their labs would be kitchen improvisations with commensurate
consequences.
He would have had a go at our "Anders Hoveland" I'm pretty sure re: some of these threads especially the title of this one. 
[Edited on 15-7-2010 by quicksilver]
|
|
|
br25
Harmless

Posts: 3
Registered: 1-7-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by br25  | | nitromethan with 80%+ H2O2 ist very powerfull and NM with TNM.Its possible to mix NM with ammonium persulfate or isopropyl nitrate?
|
i tested NM+H2O2 95% 4,7ml NM mixed with 4ml 95%H2O2 its stable an burns very fast when heated with flame
it can be detonated with a hammer blow
NM-40% OB and pure H2O2 +47%OB
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
4-nitro,5,6-triazolo-1,2,3-triazine-2,7-N-dioxide
Molecular Formula C3N7HO4
NTTO is a hypothetical target molecule which is extremely likely to be significantly more powerful than HMX, with less sensitivity. Although HMX
consists of bigger molecules, NTTO is likely to have closer molecular packing because the molecule is much more polar, and because of hydrogen
bonding. The aromaticity and electron-donation from the NH group to all four oxygen atoms would be expected to provide molecular stability and reduce
sensitivity.
NTTO may possibly even approach the calculated power of DTTO, meaning it could exceed the power of octonitrocubane. There are structural similarities
between NTTO and DTTO, and the molecular formulas, C3N7HO4 and C2N8O4 respectively, are also somewhat similar. The structure of NTTO may also be
compared with LLM-116 (4-amino-3,5-dinitropyrazole), which has been calculated to be 90% as powerful as HMX, and has the formula C3N5H3O4, with two
less nitrogen atoms and two more hydrogen than NTTO.
NTTO may hold promise as an excellent new high-performance energetic compound.
expected decomposition
C3N7HO4 --> (2½)CO + (½)CO2 + (½)H2O + (3½)N2
Possible Preparation
Glyoxal is condensed, under alkaline solution, with a limited quantity of nitromethane to form
O=CHCH(OH)CH2NO2.
This is then oxidized to
O=CHCH(=O)CH2NO2. A molar excess of the resulting product is then reacted with sodium hypochlorite solution to obtain
O=CHCH(=O)CH2ClNO2, with a chlorine atom added to carbon atom with the nitro group.
The O=CHCH(=O)CH2ClNO2 is reacted first with sodium azide, then with an alcoholic solution of ammonia (without water), and simultaneously cyclized in
a "one-pot" reaction.
NH=CHC(NH2)=C(NO2)(N3) forms as an intermediate before transforming into 4,5-amino-6-nitro-1,2,3-triazine through a Michael-type cyclization reaction.
Shevelev obtained 4-methyl,5-nitro-1,2,3-triazole in a similar reaction from the condensation of acetaldehyde with ethyl-2,2-dinitroacetate in the
presence of sodium azide. (Shevelev used this as the precursor to 4,5-dinitro-1,2,3-triazole).
The one of the two vicinal amino groups of the 4,5-amino-6-nitro-1,2,3-triazine can be diazotized, with acidified sodium nitrite, and then cylized to
form the adjoining triazolo ring. The 4-nitro,5,6-triazolo-1,2,3-triazine thus prepared may then be oxidized by potassium persulfate to form the final
product, 4-nitro,5,6-triazolo-1,2,3-triazine-2,7-N-dioxide. This is not actually a technical name, as there is not really a "7-position" unless the
compound were described as "bicyclo-hexaazo-nonane-tetraene", the N-oxide in the triazolo add-on ring not being on the nitrogen viscinal [adjacent] to
the nitro group.
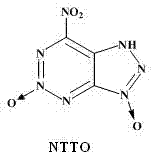
[Edited on 27-6-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
Please provide references to published or similar reactions for the various steps of this synthesis. You have already done so for some of the steps,
and I appreciate that. I would also like to see any calculations or models used to estimate the material's performance.
PGP Key and corresponding e-mail address
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
http://en.wikipedia.org/wiki/Nitroaldol_reaction
looks as though condensation of nitromethane with glyoxal results in cylization, so it may be better to start with glycolaldehyde instead, then
oxidize both hydroxy groups to aldehyde after condensation. Unfortunately, this is going to mean low yields (since two hydroxy groups are being
oxidized).
2-Iodoxybenzoic acid might be the ideal regent for the selective oxidation, but then one would not want to employ DMSO as the solvent as it would
result in cleavage of the vicinal aldehyde.
http://onlinelibrary.wiley.com/doi/10.1002/anie.196402111/ab...
"...solution of chloronitromethane (ClCH2N02) was formed. Apparently the reactivity of chloronitromethane toward hypochlorite is not greater than
nitromethane"
"Exhaustive chlorination of nitromethane under alkaline conditions yields chloropicrin (trichloronitromethane, Cl3CNO2)"
"nitromethane...Other processes for the formation of chloropicrin require an excess of sodium hypochlorite or other alkaline hypochlorite."
http://pubs.acs.org/doi/abs/10.1021/ja00522a050
http://www.springerlink.com/content/w8wt6n4063384875/
Diazotization of an amino group can allow it to link up to another amino group, in the case is vicinal amino groups, this would likely lead to
cyclization to form a triazolo derivitive, unfortunately cannot find a specific reference now
Look at steps (7) and (8),
https://sites.google.com/site/energeticchemical/benzene-tria...
this forum has also discussed diazotization of 5-aminotetrazole, which leades to a --N=N--NH-- bridge.
not really related, but also found this:
http://onlinelibrary.wiley.com/doi/10.1002/qua.22517/abstrac...
As for oxidation in the final step to N-oxides, even 5-nitrotetrazole can be oxidized to the N-oxide using readily obtainable "Oxone"
http://pubs.acs.org/doi/abs/10.1021/ja106892a
The exact sites of oxidation a dependant upon the best locations for electron donation
energetic performance, comparisons with related compound
DTTO has been calculated to generate pressures of 558-567 kbar, and that is the downward revised calculation, because the previous ones were thought
to be unrealistically high.
For comparison, HMX produces only 346 kbar of pressure, and octonitrocubane has been calculated at 489 kbar.
http://dodreports.com/pdf/ada513104.pdf
oxidation of amines
Also want to comment on an earlier post, reaction of secondary amines with H2O2 produces nitrones.
For example, CH3CH2CHNHCH2CH2CH3 is oxidized to
CH3CH2CH=N(-->O)CH2CH2CH3 in 89% yield.
(where the compound is an N-oxide, there are not any carbon-oxygen bonds)
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv9...
http://www.sciencedirect.com/science/article/pii/00404039950...
http://docs.google.com/viewer?a=v&q=cache:bNaeoQOt0koJ:w...
It appears that a catalyst is required, whether tungstate, selenium dioxide, or titanium-silicate zeolite.
apparently H2O2 can be used to oxidize methylamine to
formaldoxime CH2=NOH
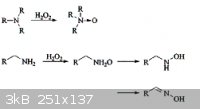
[Edited on 27-6-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
just some ideas-
http://www.shadowrx.com/forums/showthread.php?t=1505
[Edited on 29-6-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
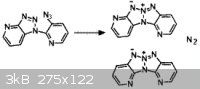
R.A. Carboni, J.C. Kauer, J. American Chem. Society, volume 89, p2633, (1967).
Based on the above reaction which is described in literature, perhaps the reaction below would also work...
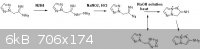
The final product would be 4-amino-pyrrolo[2,1-c]-1,2,3-triazole, which could be a useful scaffold. Presumably, it could be easily nitrated.
Although unsubstituted 1,2,3-triazole is impossible to directly nitrate, that amino group should activate the molecule to easy to nitration.
4-nitro-1,2,3-triazole, for example, is not only easily nitrated, but it is also simultaneously oxidized under the mixed-acid nitration conditions to
4,5-dinitro-1,2,3-triazole-1N-oxide.
That is to say that 4-amino-pyrrolo[2,1-c]-1,2,3-triazole would probably add three nitro groups under normal nitration conditions,
without much difficulty.
The intermediate 2-[2N-connected-1,2,3-trazinyl]-acetamidinyl azide, with the structure
(H2C2N3)CH2C(=NH)N3, would doubtless reversibly cyclize into the tetrazole. The alkaline conditions both allow the cyclization, and allow it to
hydrolyze back. This side equilibrium should not effect final yields. Just boil the alkaline solution until nitrogen gas ceases to be liberated.
[Edited on 1-8-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv4...
states that the reaction of 2-nitroaniline with hypochlorite solution produces "benzofurazan oxide" (more commonly named benzofuroxan).
Could the explosive "Fox-7" be similarly converted (by reaction with NaOCl) to 3-amino-4-nitro furoxan?
The actual chemical name for Fox-7 is 1,1-diamino-2,2-dinitroethylene.
Would further oxidation by the hypochlorite bridge the two amino groups, to yield 4,4’-Dinitro-3,3’-diazenofuroxan (DDF)?
If this route is feasible, it could potentially be a very direct and convenient way to prepare DDF, since the commercial availability of Fox-7 is
increasing as a specialty research propellent. A few members in this forum have actually prepared Fox-7.
DDF molecular structure:
NO2-(C2N2O2)-N=N-(C2N2O4)-NO2,
where (C2N2O4) represents a furoxan ring.

Wikipedia describes DDF as having an extremely high detonation velocity around 10,000 m/sec, with a density of
2.02 g/cm3.
From another source (translated from russian literature):
Dinitroazofuroxan (formula C4N8O8 ) is thermally unstable, has a detonation pressure of 460kbar, and a detonation velocity of 9.7 km/sec at 1.94
g/cm3. The compound was fairly sensitive, but not extremely so.
"Dinitroazofuroxan" is just another simplified name for the 4,4’-Dinitro-3,3’-diazenofuroxan (DDF).
[Edited on 8-8-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Having actually seen the the original reference, the 10,000 m/sec detonation velocity that wikipedia states for DDF is certainly not a precise
measurement or calculation.
It is quite possible that the crystal density of the compound is actually 2.02 g/cm3, as wikipedia states. Sometimes, in these sorts of situations,
there exist more than one measured density value for the same compound in the literature. Usually the larger value is correct, as often the substance
being measured was not in its most compact form. One really cannot blame researchers for not wanting to work with a big sample size, then boil out all
the solvent and squeeze it down to get rid of potential little air bubbles, when the substance could likely explode! Another problem is that some of
these energetic compounds have thermal stability problems. Trying to melt it down or boil out all traces of solvent is likely to cause some minor
decomposition, which will upset the results.
Assuming that DDF actually has a 9.7 km/sec at a density of 1.94 g/cm3, and assuming its actual crystal density is 2.02 g/cm3, a calculation can be
made.
√(9.7^2 * 2.02/1.94 ) = 9.89797959
that is to say that the square of 9.7 times 2.02, divided by 1.94 is 97.97. then the square root of that is 9.89797959.
you might wonder why the 9.7 was squared, and then the square root was later taken. This is because energy is proportional to the square of
velocity, the relationship is not linear. 9.7 * 2.02/1.94 would actually have given an even higher value of 10.1.
If the values quoted in the literature from both sources are correct, which is a big assumption, then DDF actually has a detonation velocity of about
9,898 meters per second, in its densest form. Remember, this value should not be taken as a precise calculation, but rather as an
indicative estimate.
These sorts of calculations can be used to help fill in gaps in knowledge about obscure explosives, for which there is little available research.
Please feel free to share your own comments or opinions about this calculation.
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Fox-7 can be used as a precursor for other energetic compounds.
Cannot find any references now, does anyone else have information about this?
Read that Fox-7 could be used to make dinitromethyltetrazole, although other better routes exist.
The picture below just shows what was discussed in the last two posts, the proposed oxidation of Fox-7 to DDF. Some of the members might not be able
to understand the chemical naming terminology without seeing a picture.
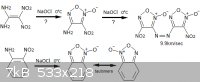
As to the tautomerism of benzofuroxan, it is unclear whether other furoxan derivitives (such as DDF) would also show the same tautomerism,
http://www.icts.uiowa.edu/Loki/publications/browsePublicatio...
[Edited on 11-8-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
| Quote: |
Secondary alkylamines are readily oxidized to hydroxylamines by hydrogen peroxide at room temperature, but yields of hydroxylamine are usually low due
to further oxidation.
|
The paper goes on to say that the further oxidation can be prevented if the alkylamine is complexed to cobalt(III) ions.
"Synthesis and characterization of encapsulated cobalt(III) hydroxylamine complexes", Daryl J. Bull, Inge I. Creaser, Alan M. Sargeson, Brian W.
Skelton, Allan H. White
Inorg. Chem., 1987, 26 (18), pp 3040–3043
So potentially, dimethylamine could be directly oxidized to dimethyl hydroxylamine if there are cobalt ions present (the cobalt(II), in the presence
of amine, would readily be oxidized to cobalt(III) in the presence of air (or H2O2) and some acid (dimethylamine nitrate for example).
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Off topic, but could you think of any possible energetics easily derived from diketopiperazine? I just noticed it can be easily made from glycine...
And maybe even use other amino acids to allow other possibilities. The dinitrate ester of the n,n-dinitropiperazine formed from serine maybe? Or
histidine? Lots of options...
[Edited on 21-2-2012 by 497]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I really think that dimethylhydroxylamine nitroformate would be a very powerful compound with relatively low sensitivity. But it would probably not be
good to directly add free nitroform to dimethylamine because free trinitromethane is a reactive oxidizer, for example oxidizing ferrous ions Fe+2 to
ferric Fe+3. The nitromethanate ion, however, is not as oxidizing as free trinitromethane, as evidenced by the stability of the nitroformate salt of
hydrazine. So a better route would be, for example, reacting dimethylhydroxylamine sulfate with aqueous calcium nitroformate, precipitating out the
insoluble CaSO4, and then letting the remaining solution evaporate, leaving crystals of the desired compound.
Dimethylhydrazine nitroformate, for example, has excellent explosive performance, and the sensitivity is not too high.
http://nopr.niscair.res.in/bitstream/123456789/8624/1/IJCT%2...
| Quote: |
MonoMethyl Hydrazinium Nitroformate has a det. velocity of 9.134km/sec. This compares with 8.93 for RDX. MMHNF is also 212% more powerful than TNT on
a weight basis, compared with 163% for RDX. The two compounds have similar sensitivity.
Journal of Chem. Tech. Vol12 2005
"Synth, Characterization, and thermal behaviour of hydrazinium nitroformate..."
H.S. Jadhav, M.B. Talawar (India)
|
Hydroxylamine nitroformate would probably be even less sensitive because there are no hydrogen atoms on the nitrogen atom of the hydroxylamine group,
so it would be much less vulnerable to oxidation than other hydroxylamine compounds. Reactive reducing agents, such as hydrazine and hydroxylamine,
tend to significantly increase the sensitivity of explosives.
hydroxylamine nitroformate:
HONH[+](CH3)2, C(NO2)3[-]
[Edited on 26-2-2012 by AndersHoveland]
|
|
|
| Pages:
1
2 |
|