Fischer
Harmless

Posts: 3
Registered: 16-5-2010
Member Is Offline
Mood: No Mood
|
|
Bromination of benzodioxole
Hello everyone!
I have been reading a book called "Total Synthesis II" and in it is described how a carbon string is attached to Benzodioxole by first substituting a bromine atom to it. The final result of this is suppose to be 5-(2-propenyl)-1,3-benzodioxole.
However my problems is that the Bromo-Benzodioxole's bp is so high that, according to the book, a 10mmHg vacuum is needed to distill it, or otherwise it will "fry". The vacuum I
have is only 75mmHg. Can anyone recommend a way around this? Preferably one that will work!
Thanks!
[Edited on 16-5-2010 by Fischer]
[Edited on 16-5-2010 by Fischer]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
A) get a better vacuum pump, one that can pull down to 1 mmHg or better, and a cold trap and vac regulator.
B) steam distill, which will not allow much fractionation between benzodioxole and the methylenedioxybromobenzene, but will separate them from tars.
The pump you have will do fine with removing solvent and traces of Br2, both of which are better done with an aspirator anyway as with the water pump
there's no worry of contaminating the pump oil.
As Strike says, you need serious vacuum.
BTW - you mistitled the thread, since it is nit benzene you're brominating and which would both require a different method of bromination and
different work-up. On top of that you failed to provide a decent reference to the procedure, some searching should turn up journal references to the
procedure
[Edited on 16-5-2010 by not_important]
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
| Quote: | Can anyone recommend a way around this? Preferably one that will work!
|
Just a suggestion and not really a good one but possibly hydrolysis of the Bromobenzodioxole with excess NaOH which I would assume would form the
Sodium Phenolic salt, more then likely a solid, and remove the other materials like benzodioxole with a solvent like Dichloromethane. Once purified
rebrominate it back to Bromobenzodioxole.
Hell of a work around to avoid getting a stronger vaccume pump though.
My worry is that if your looking for "workarounds" this early in an Ecstacy synthesis someones going to get hurt.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Fischer
Harmless

Posts: 3
Registered: 16-5-2010
Member Is Offline
Mood: No Mood
|
|
Alright, I edited the tittle.
Im not so much looking for "workarounds" as planning ahead for back up plans. I havent been able to find a pump that could pull below 10mmHg and would
cost less than 1000e. (I live in the EU)
Also I tried to be a bit secretive since I didn't know how people here react to this topic. I have already found some articles about going around the
distillation of a halogenic substance. Witch afaik is the only reason one would need a strong vacuum. Strike says at the beginning of his book that
"any commercially availible pump is ok" and when it comes to distilling the bromo-benzodioxole, I dont think he mentiones anything about it being
decomposed at low temperature, thus needing a good vacuum. The guy who says this is his co-writer.
Here's what I have found:
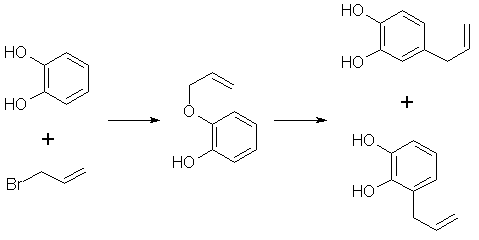
1. Here whit allylbromide added to cathecol. See picture below.
However, this page is the only one that mentiones this method. So, I dont know if it is away of getting around of buying the expensive pump.
And 2nd in the book "Total Synthesis II" there is a short description at page 244. In it allyl chloride is added whit small amount of powdered Cu to
benzodioxole.
What I dont understand is why Strike would write so little about it, it only takes one page and looks very easy compared to the Grignard reaction
method (the one whit brominated benzodioxole getting distilled). He has a habit to hype things in the book. And he doesnt do to whit this method.
Witch makes me wonder if it will work.
Im unfamiliar whit steam distillation, but Ill keep this possibility in mind.
[Edited on 16-5-2010 by Fischer]
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Quote: Originally posted by Fischer  |
Also I tried to be a bit secretive since I didn't know how people here react to this topic. ....Im unfamiliar whit steam distillation, but Ill keep
this possibility in mind. |
Hmmm. Are you interested in chemistry or making a consumer product? Why the need for secretiveness? Sounds as if your chemistry is as poor as your
spelling.
I can't find this journal "Strike" that is your primary reference.
[Edited on 16-5-2010 by entropy51]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Fischer  | | However my problems is that the Bromo-Benzodioxole's bp is so high that, according to the book, a 10mmHg vacuum is needed to distill it, or otherwise it will "fry". The vacuum I
have is only 75mmHg. Can anyone recommend a way around this? Preferably one that will work! |
I'm sorry, but someone who is so poorly educated in practical chemistry that he never even heard about aspirators and furthermore uses Strike's book
and Wikipedia as his final references instead of checking the primary literature, is certainly not going to be able of doing any such multistep
synthesis (at least not without injuries and mishaps). I suggests to you to either start learning chemistry (properly) or give up now. If your only
motivation is greed you will get nowhere anyway, because with money obfuscating your mind you will never be able to learn or understand anything.
Chemistry does not work when using recipes. Yet, if your motivation is based on the passion for science, then rather start a proper learning process
and then maybe someday you will be able to synthesize not only MDMA, but also much better and legal drugs.
PS: Meanwhile, for beginners questions like stuff about vacuum distillation, use the Beginners section of the forum (where I'm moving this thread).
There we also have several good threads about vacuum distillation and fractionation that you should have checked first.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Nicodem
|
Thread Moved
16-5-2010 at 23:44 |
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
this thread is probably better suited for thevespiary 
difference in objectives thats all.
e3500 console login: root
bash-2.05#
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Possibly your right Ephoton but it would also suck for Thevespiary to become a catching net for all those just looking into making drugs and know
nothing about chemisty itself.
Nicodem said it best himself a while back (<- hows that for ass kissing ),
that if you learn mechanisms many questions won't need to be asked. I still wounder where the chem world would be today if people asked about the
mechanics of a secondary alcohol reduction using HI/rP instead of asking " How doz I mak Mef". ),
that if you learn mechanisms many questions won't need to be asked. I still wounder where the chem world would be today if people asked about the
mechanics of a secondary alcohol reduction using HI/rP instead of asking " How doz I mak Mef".
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Sort of following along - not knowing what steam distillation is puts you in a similar situation as someone using a cookbook that has a section on
preparing a 9 course dinner but is stopped by the phrase "grate the cheese" because they don't know how to grate cheese or even what that means.
Note that the yields from the copper based route are maybe 30%, and it creates a yucky mess that has to be worked up.
An understanding of the basic is really helpful, both the "why" and "how-to" learned may mean you don't get stumped at some point where things didn't
turn out just as expected to, or you are lacking some reagent or apparatus.
|
|
|
Fischer
Harmless

Posts: 3
Registered: 16-5-2010
Member Is Offline
Mood: No Mood
|
|
I'm not motivated only by greed. Iv been studying chemistry for awhile now, and I'm going to uni next semester. But I haven't taken any lab.
Unfortunately, there does not seem to be any schools near by that would offer a lab course during summer. Note that I only asked advise whit safrole
synthesis and not the whole MDMA. Also rest assured that I am taking safety seriously and Iv invested a lot money to it.
|
|
|