coccoc
Harmless

Posts: 24
Registered: 21-9-2009
Member Is Offline
Mood: No Mood
|
|
Reaction in Thunder in a Testube?
Can you suggest how the reactants react in the experiment of thunder in a testube?
When Acetone is used, Permanganate contacted with conc. sulfuric acid to form Manganese Heptoxide, which then reacts, with acetone producing red oily
liquid.
So how many reactions may actually occur in this experiment?
|
|
|
woelen
Super Administrator
        
Posts: 8013
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I have done this experiment with ethanol, sulphuric acid and KMnO4. It probably also works with acetone, isopropyl alcohol or propyl alcohol.
http://woelen.homescience.net/science/chem/exps/mini-expl/in...
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
I think you might fairly consider every possible oxidation of the acetone molecule. I'm no expert on metals, but I imagine Mn2O7 behaves a bit like
MnO4(-) or HCrO4(-). Consider the enol tautomer of acetone, and you can write an array of mechanisms to "reduce the manganese" and cleave and oxidize
acetone to formic acid and acetic acid. I've proposed some manganese species that perhaps an inorganic chemist wouldn't like, but I imagine they could
react further. I couldn't get MnO2 out of a mechanism  oh well. My answer to
your question would be that there are a LOT of competing reactions going on, both in manganese species present and with regards to acetone. But here
are some ideas: oh well. My answer to
your question would be that there are a LOT of competing reactions going on, both in manganese species present and with regards to acetone. But here
are some ideas:
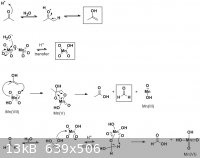
[Edited on 8-11-2009 by Arrhenius]
|
|
|
coccoc
Harmless

Posts: 24
Registered: 21-9-2009
Member Is Offline
Mood: No Mood
|
|
I have also done this experiment and make a video, I just wonder if what I think about the reactions is correct, thanks for the data.
|
|
|
coccoc
Harmless

Posts: 24
Registered: 21-9-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Arrhenius  | I think you might fairly consider every possible oxidation of the acetone molecule. I'm no expert on metals, but I imagine Mn2O7 behaves a bit like
MnO4(-) or HCrO4(-). Consider the enol tautomer of acetone, and you can write an array of mechanisms to "reduce the manganese" and cleave and oxidize
acetone to formic acid and acetic acid. I've proposed some manganese species that perhaps an inorganic chemist wouldn't like, but I imagine they could
react further. I couldn't get MnO2 out of a mechanism  oh well. My answer to
your question would be that there are a LOT of competing reactions going on, both in manganese species present and with regards to acetone. But here
are some ideas: oh well. My answer to
your question would be that there are a LOT of competing reactions going on, both in manganese species present and with regards to acetone. But here
are some ideas:
[Edited on 8-11-2009 by Arrhenius] |
The mechanism is quite good, and I think there is some MnO2 although I don't exactly know the mechanism.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
I wonder if it is presumptuous to think it only oxidizes the organics to their respective carboxylic acids?
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
CO2 is the only higher oxidation state of carbon here, so sure, it's possible. Is that what you mean? However, it doesn't look like there's very much
gas evolving.
|
|
|