Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
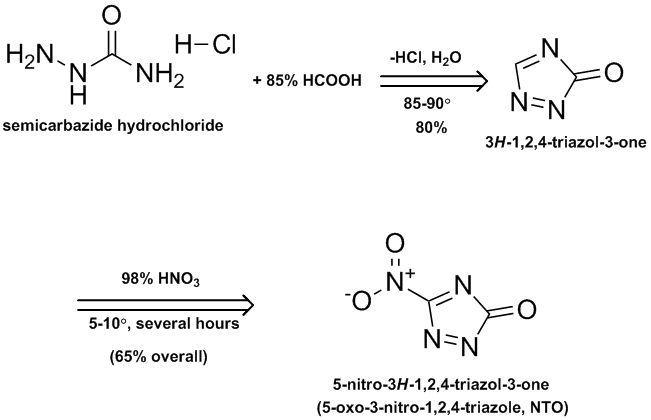
5-nitro-3H-1,2,4-triazol-3-one (NTO)
This secondary explosive is disclosed in patent US5034072 to the French explosives & munitions company SNPE. Its performance is described as
follows:
detonation velocity = 7700 m/s p= 171 gm/cm^3
(8950 m/s @ p = 1.91 gm/cm^3)
impact sensitivity = 22 J
friction sensitivity 7% @ 353 N
It is further described as having explosive power nearly that of hexogen without having the sensitivity of either hexogen or octogen.
See http://www.pat2pdf.org/patents/pat5034072.pdf.
[Edited on 26-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Axt
National Hazard
   
Posts: 814
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
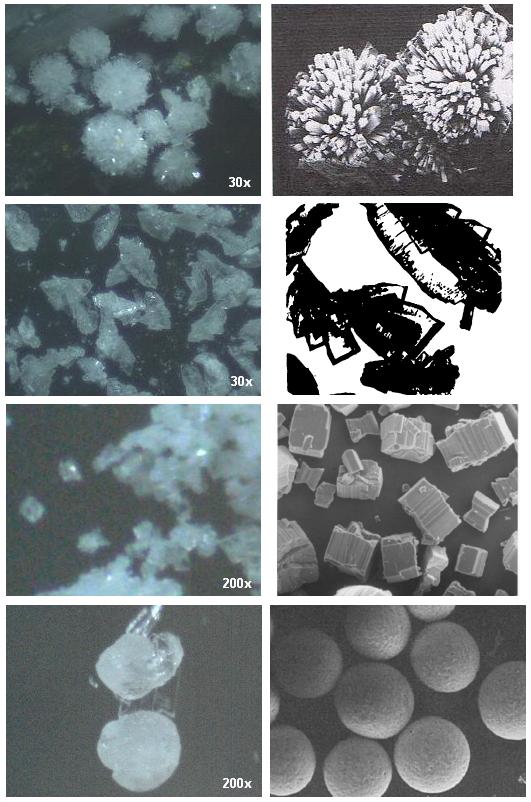
I've prepared NTO via hydrazine + urea -> semicarbazide, glycerine + oxalic acid -> formic acid, semicarbazide + formic acid -> triazolone,
triazolone + 70% HNO3 -> NTO.
Its an interesting explosive to play around with crystalisation, the attached picture shows my crystals on left and those from literature on right
using different crystalisation methods.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Axt
Its an interesting explosive to play around with crystalisation, the attached picture shows my crystals on left and those from literature on right
using different crystalisation methods. |
Very nice! Are these polymorphs? Many materials are capable of existing in a large number of discrete crystalline states, especially when you start
adding in molecules of the solvents used in the crystallization.
[Edited on 26-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Axt
National Hazard
   
Posts: 814
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Just different crystal forms, I really dont know if different polymorphs are being formed and which is which. NTO does have a number of polymorphs.
The first formed a part of the initial product of nitration, which could be separated by "gold panning" them out as they are quite large and roll
easily.
Second was simple crystallisation from water.
Third is the most dense form, small cubes. I used a vibrator attached to the outside of a glass beaker, recrystallised from water. In the literature
they used rapid stirring, ultrasound or both.
The spheres in last picture used alcohol, though cant remember the exact method. It was quite hard to get them though. Investigated in the literature
as easily flowing form and for casting with TNT.
[Edited on 27-7-2008 by Axt]

|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
NTO also forms salts: e.g. hydrazine (HNTO), methylamine, aminoguanidines, etc. (US 5256792). And estimated VOD of 3-nitro-1,2,4-triazol-5-one (NTO)
is 8590 m/s at d= 1.91. Using this patent's thermodynamic data, I got with HNTO an estimate of about 7340 m/s at 1.65, and where NTO was 8240 m/s at
1.91.
|
|
|
TechnologicallyRetarded
Harmless

Posts: 13
Registered: 14-8-2009
Member Is Offline
Mood: No Mood
|
|
Axt, I notice that you produced Semicarbazide from Hydrazine and Urea. I was searching for the synthesis of Aminoguanidine from the previously
mentioned reagents, thinking they would produce a 'Hydrazone'.
So am I right in thinking that the amino groups are nucleophilic enough to render the oxo group resistant to further nucleophilic attack? Would
different conditions favour the production of a Hydrazone?
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
Well, semicarbazide looks just like an aminated urea. Hydrazine is a good nucleophile, so maybe the amino group kicked out a hydrogen?
@Axt: Did you use anhydrous hydrazine and urea?
Anyone done a test on it yet?
|
|
|