| Pages:
1
2
3
4 |
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
.............perhaps I should read my own posts sometimes........
Make no mistake, the ones I made were definitely conconducting ® ones 
DLC on Si below:
Dann2
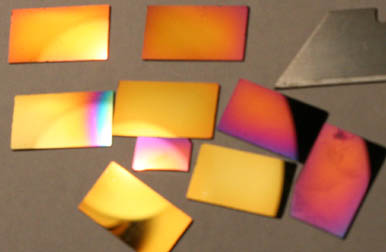
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
Hello Dann2,
I'm not sure exactly what you're getting at when you say that DLC films "[are] not the same as diamond".
Anyway, there is a brief general explanation of DLC here.
As far as the conductivity goes, you're right, diamond itself (except for "type IIB") and straight DLC films will be nonconducting. We would need to
add a boron compound to the electrolyte to solve that problem.
As soon as I can get my hands on a suitable HV power supply, I plan on doing some experiments.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
DLC (Amorphous, 7 types) versus Crystaline (one type??) Diamond coat
Hello JP,
| Quote: | Originally posted by jpsmith123
I'm not sure exactly what you're getting at when you say that DLC films "[are] not the same as diamond".
....................
|
As far as I can see there seems to be DLC (7 different forms all amorphous) and then Diamond films (crystalline)
Quote from here.
"Diamond-like carbon (DLC) is an umbrella term that refers to 7 forms of amorphous carbon materials that display some of the unique properties of
natural diamond. The German Fraunhofer - IST institute has organized them into the chart form seen in this page background."
The chart here seperates the DLC from the 'Diamond films'.
Perhaps I am splitting hairs but I think you need the actual Diamond films (not amorphour) for Electrodes.
Perhaps as you say doping DLC will make it suitable.
The 'Diamond film' (crystaline not amorphous) needs to be doped for conductivity.
It should be noted that I know little or nothing regarding the DLC/Diamond coat stuff so I am close to guessing and perhaps splitting hairs.
The Diamond stuff is definitely an interesting avenue.
Did you try to obtain the Thesis (way above) regarding Acetic + HV supply?
I emailed the Author + email at bottom of Thesis page just now. Will see if they reply.
edit:
Attached a paper from Ref. Section.
Hope I am not clogging up the board with papers or should I link to it in the ref. sec. instead of attaching?
It is not a very hands-on or 'useful to us' paper me thinks.
HAVE A GOOD (BEDTIME) READ ANYWAYS!!!!!!!!!!
Cheers,
Dann2
[Edited on 10-2-2009 by dann2]
Attachment: Diamond on Ti for ElectroChem.pdf (1.4MB)
This file has been downloaded 962 times
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by jpsmith123
As soon as I can get my hands on a suitable HV power supply, I plan on doing some experiments. |
Well, check this one out:
http://cgi.ebay.ca/Hipotronics-HV-DC-Power-Supply-850-30-Hig...
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
@Dann2
From all I've read I think what we're concerned with is the "quality" of the film (i.e., the relative amounts of SP3 vs SP2 bonded carbon), the
adhesion to the substrate, and the conductivity.
The paper recently posted here regarding electrodeposition of DLC films from ethanol seems to demonstrate a relatively "high quality" film (apparently
comparable to that of plasma or hot filament CVD deposition methods), at 300 volts and millimeter spacing.
Moreover, from all I've read, it seems that doping with boron, in addition to increasing the conductivity, will also tend to increase the quality, the
adhesion, and the deposition rate of the film as well.
The main question I have right now is: What substrate do we use? Several papers (including the one you've recently posted) point out the difficulty
encountered with putting DLC on Ti, but these papers have all been concerned with CVD methods where the substrate is coated at a high temperature. We
may not have a problem putting DLC film on Ti at room temperature. Also, what about if the Ti surface is hydrided?
Lastly, if electrodeposition methods can build up a coating rapidly (as I would think, especially since they're presumably being made conductive with
boron doping) maybe we don't need a substrate? If it's possible to make a piece thick enough to stand alone, maybe an electrical contact can be
electroplated onto it?
@Tentacles
Although the specs for that supply aren't explicitly stated in the ad, in looking at the panel meters, it seems the voltage is too high and the
current too low. I think a max voltage in the range of 500 to 1000 volts at 100 ma or so current would be ideal.
|
|
|
Twospoons
International Hazard
    
Posts: 1302
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
Why not the obvious substrate : graphite !
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by jpsmith123
Although the specs for that supply aren't explicitly stated in the ad, in looking at the panel meters, it seems the voltage is too high and the
current too low. I think a max voltage in the range of 500 to 1000 volts at 100 ma or so current would be ideal. |
You should look for electrophoresis power supplies! I picked up an LKB 2103 a few months ago, it was about NZ$100 (US$50) and although about 25 years
old is in excellent condition. They would be cheaper in the US for sure.
It has constant power, voltage and current (110W, 0-2KV and 0-200mA) ideal for high voltage electrolysis.
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
Hello Xenoid,
I've actually been looking for electrophoresis power supplies. I've seen some units manufactured by "ISCO" (e.g., model #493, #494) for sale at some
surplus places (online), but I can't find any specs, user manuals, etc. for them.
BTW, have you tried electrolyzing any methanol, ethanol etc., at high voltage yet?
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
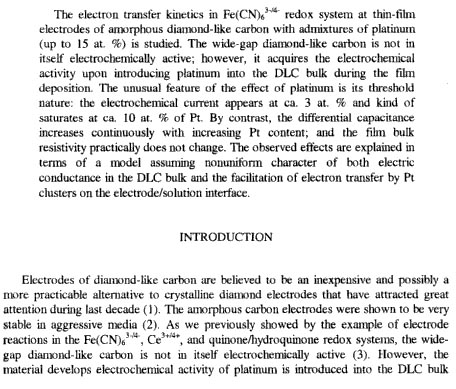
See here for some info. on DLC with Pt in it. They seem to say that DLC is not electrochemically active (like it cousin crystalline diamond).
The info. is on page 108
See also page 97 (same book) for some stuff on BDD.
And page 88 and page 72............
FFS Dann2 just post the whole book!!!!!!!!!!!
Sorry but I am scanning through book and posting at the same time.
To sum up> This book is worth taking a look at.
Wonder can anyone over in the (glorious, heroic and galant) Ref. Section get this book.
Intro. (from page 108) in jpeg below.
Dann2
[Edited on 12-2-2009 by dann2]
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
Hmmm I'm not sure I understand precisely what they mean. Are they merely pointing out that straight amorphous DLC is not a conductor...ipso facto it
is not "electrochemically active"? But crystalline diamond is also an insulator, no? I'm confused.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello JP,
Don't know I will have to say.
See here:
http://materials.web.psi.ch/Publications/Publ_MatDev_files/2...
Section 22.5 page 581
It seems to say that DLC is electrochemically active. It's info. from 1997. They are only referring to DLC being used as electrodes as opposed to an
Anode for actually making stuff.
Thesis here:
http://materials.web.psi.ch/Publications/Publ_MatDev_files/T...
Not much use to us (after having a look at it). There is some rather complicated machinery for DLC coating ..............
Patent here:
here saying (examples close to bottom):
"The DLC can be made such that it is more electrically conductive by changing the deposition conditions, thereby making the DLC coated mesh useful as
an electrode for electrochemical applications. Additionally, DLC can be doped with boron, phosphorus, other metals and non-metals."
Google search here
second from bottom:
...... Thus, we conclude that DLC is electrochemically inactive in itself.........
Will look for this paper (book?) in ref. section.
EDIT:
Link to this book here
Thanks to gsd for that one.
Attached is a paper on DLC Films doped with Phosphorous
Dann2
a rather rambling post
[Edited on 13-2-2009 by dann2]
[Edited on 13-2-2009 by dann2]
Attachment: P doped dlc.pdf (460kB)
This file has been downloaded 1420 times
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
Well Dann2 I find that the more papers I look at, the more confused I become.
IMO the most promising paper so far is the one by Sreejith et al. regarding the "low voltage" deposition of DLC from ethanol, where the resulting SP2
fraction was 7.1% (@ 300 volts and 1 mm spacing). However it would be nice to see somebody else duplicate these results.
Anyway, assuming that adding some ethyl or methyl borate or even a little boric acid would dope the DLC with boron and make it conductive, well this
might be just what we're looking for.
As I see it the biggest unknown is the choice of substrate material...I think we're at the point where some experimenting is in order...now if I could
just find a decent yet cheap adjustable HV power supply...
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Hi,
just to add my two cents on references:
http://www.scielo.br/pdf/jbchs/v17n4/30537.pdf
http://www.scielo.br/pdf/mr/v2n2/0016.pdf
http://www.scielo.br/pdf/qn/v28n2/23655.pdf
http://www.scielo.br/pdf/jbchs/v15n1/a04v15n1.pdf
I wanted right now to attach the thesis "Estudos da eletroquímica do diamante dopado com boro e da sua superfície modificada com catalisadores para
a oxidação de metanol e etanol" ("Studies of boron-doped diamond eletrochemistry and of their surface modified with catalyst for the methanol and
ethanol oxidation" - with english abstract), but it seems too big and painfull slow to load, so to get the pdf go to: http://www.teses.usp.br/teses/disponiveis/75/75131/tde-11042... , click on "GiancarloRSalazarBanda.pdf " in the bottom , mark the box "Eu li e
concordo com as condições acima." ("I have read and agreed with abovementioned conditions") and then in "Condordo".
If anyone wants a quick translation of any part in these portuguese documents, let me know although I dont guarantee "100%" in translation since there
are very technical terms in portuguese in these documents that are very hard to find a equivalent in english speaking.
[Edited on 15-2-2009 by Aqua_Fortis_100%]
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
Found something that could be interesting, I have no idea if Lonsdaelite is conductive but this could be interesting to look at.
http://en.wikipedia.org/wiki/Poly_(hydridocarbyne)
A polymer which take a Diamond-Like form when heated to 110°C under Argon at standard pressure.
Interestingly it can be synthesized by electrolysis of chloroform.
/}/_//|//) /-\\/|//¬/=/_
My PGP Key Fingerprint: D4EA A609 55E4 7ADD 8529 359D D6E2 33F6 4C76 78ED |
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
@Blind Angel:
Thanks for that information...I've never heard of anything like that before. It's fascinating. I wonder how easily it could be boron-doped?
@Everyone:
I found an online "book" about boron-doped diamond electrodes, wherein the author implies that, for purposes of electrochemistry, the graphitic (SP2) carbon content
in the DLC film should be < 1.0%...lest conduction in the unwanted graphite "short circuit" the active diamond surface. If this is actually the
case, then it seems that all the electrodeposition methods known to date are inadequate.
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
Here's an interesting paper to read.
I wonder what boron compounds would be the best candidates to try to dope it with?
Attachment: Facile Synthesis of Poly(hydridocarbyne) A Precursor to Diamond and Diamond-like Ceramics.pdf (176kB)
This file has been downloaded 2286 times
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Came accross the following attachment. Might be worth a read.
Dann2
Attachment: Doped Diamond, A compact review.pdf (804kB)
This file has been downloaded 702 times
|
|
|
Hydragyrum
Hazard to Self
 
Posts: 62
Registered: 29-4-2009
Member Is Offline
Mood: Curious
|
|
So, are electrodes of diamond-like carbon the same as glassy carbon electrodes?
My understanding of glassy carbon is that it is comprised of 100% sp2 C as far as can be detected, it is amorphous, but also conducts electricity like
graphite. As it is supposed to be impervious to most things (or to everything?) then perhaps it is amorphous graphite with a surface coating of
diamond.
In any case, has anybody here tried glassy carbon as an electrode material?
Chemistry is life (and a whole lot more)
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
I have never heard of anyone trying it. It cannot be got AFAIK.
Do know any sources? Is it expensive?
Dann2
|
|
|
Hydragyrum
Hazard to Self
 
Posts: 62
Registered: 29-4-2009
Member Is Offline
Mood: Curious
|
|
It can be bought here.
Some info about it is here and here. This paper (sorry, I have no access) seems to suggest that glassy carbon is composed of fullerene nanotube - well, that's consistent
with the 100% sp2 claim made elsewhere.
In fact, a large list of further info can be found here and here.
Disclaimer: I have no first-hand knowledge of this stuff, but it sure looks interesting.
Chemistry is life (and a whole lot more)
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
Hello Hydragyrum,
I have also never heard of anyone trying glassy carbon.
However there's one patent I know of, US #5131989 which mentions the use of glassy carbon as an anode in some kind of perchlorate producing cell.
According to it:
"Materials which can be employed in the anode structures include platinum and platinum group metals, metal substrates coated with platinum or platinum
group metals, platinum group metal coated substrates, glassy carbon, fluorinated carbons, lead dioxide and metal substrates coated
with lead dioxide, noble metal oxides, and metal substrates coated with noble metal oxides. Suitable metal substrates include valve metals such as
titanium and niobium among others. Especially useful as an anode is a platinum coated niobium expanded metal having, for example, 100-200 mils of
platinum metal bonded to the niobium substrate".
So it might be worth testing if you can get some at a reasonable price.
|
|
|
Hydragyrum
Hazard to Self
 
Posts: 62
Registered: 29-4-2009
Member Is Offline
Mood: Curious
|
|
Sorry, I have no direct experience... but what I have read suggests that this material is used by quite a few. If it's as impermeable as is suggested,
price is kind of irrelevant as the electrode probably won't degrade much, if at all.
Chemistry is life (and a whole lot more)
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
Well the primary question is: Will it make perchlorate efficiently or will it make oxygen? If it won't make perchlorate then it doesn't matter how
much it erodes since it's useless.
|
|
|
Hydragyrum
Hazard to Self
 
Posts: 62
Registered: 29-4-2009
Member Is Offline
Mood: Curious
|
|
Quote: Originally posted by jpsmith123  | | Well the primary question is: Will it make perchlorate efficiently or will it make oxygen? If it won't make perchlorate then it doesn't matter how
much it erodes since it's useless. |
For that particular use (making perchlorate), it may be useless (I don't know which it would produce, perchlorate or oxygen); this does not make it
useless in general.
Anyway, it is just another possibility to try.
Chemistry is life (and a whole lot more)
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hydragyrum  | Quote: Originally posted by jpsmith123  | | Well the primary question is: Will it make perchlorate efficiently or will it make oxygen? If it won't make perchlorate then it doesn't matter how
much it erodes since it's useless. |
For that particular use (making perchlorate), it may be useless (I don't know which it would produce, perchlorate or oxygen); this does not make it
useless in general.
|
If you've been following the the relevant discussions in this forum (including this thread), you'll see that we're talking specifically about finding
an alternative to Pt and PbO2 with regard to production of perchlorate.
| Quote: |
Anyway, it is just another possibility to try.
|
Well of course it is.
|
|
|
| Pages:
1
2
3
4 |