| Pages:
1
2 |
McLovin382
Harmless

Posts: 19
Registered: 26-9-2008
Member Is Offline
Mood: No Mood
|
|
Ethyl analog of Aspartame
So, Aspartame - as many know - is an artificial sweetener used in splenda and the like. Its opponents say that it can cause health problems because
the methyl group on the molecule can break off and go through the standard methanol metabolism etc.
If that is the case, why not use an ethyl ester instead of a methyl one in the synthesis of the compound? This would cause a lot less problems and I
don't see any reason why it would reduce the sweetness of the base compound.
Surely someone must've thought of this before right?
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Aspartame is described on e.g.
http://en.wikipedia.org/wiki/Aspartame
http://en.wikipedia.org/wiki/Aspartame_controversy
http://www.nzfsa.govt.nz/consumers/chemicals-nutrients-addit...
http://www.aspartame.org/
http://www.sweetpoison.com/aspartame-side-effects.html
http://www.aspartamekills.com/
http://www.holisticmed.com/aspartame/
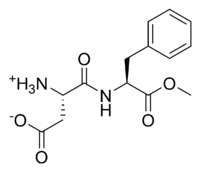
The systematic chemical name of the stuff is aspartyl-phenylalanine-1-methyl ester; being a methyl ester of the dipeptide of the amino acids
L-aspartic acid and L-phenylalanine. Under strongly acidic or alkaline conditions, aspartame may hydrolyze as with esters generally, releasing
methanol. Under more severe conditions, the peptide bonds are also hydrolyzed, resulting in the free amino acids.
Upon ingestion, aspartame breaks down into aspartic acid, phenylalanine, methanol, and further breakdown products including (from metabolism of the
methanol) formaldehyde, formic acid, and a diketopiperazine. There is controversy surrounding the rate of breakdown into these various products, and
the effects on those who consume aspartame-sweetened foods. The naturally-occurring essential amino acid phenylalanine is a health hazard to those
born with phenylketonuria (PKU), a rare inherited disease that prevents phenylalanine from being properly metabolized.
Aspartame was discovered in 1965 by James M. Schlatter, a chemist working for G.D. Searle & Company. Schlatter had synthesized aspartame while
producing an anti-ulcer drug candidate. He discovered its sweet taste when he licked his finger, which had accidentally become contaminated with
aspartame. It is sold under names like "NutraSweet".
Therefore, because it is synthetic, made with L-aspartic acid and L-phenylalanine and methanol as the starting reagents, it should easily be possible
to substitute ethanol for methanol in its synthesis, to avoid the highly toxic formaldehyde and formic acid generated on hydrolysis and metabolism of
the methanol. The synthesis is described here:
http://www.freepatentsonline.com/4656304.html (this patent has long since expired)
http://www.freepatentsonline.com/4549987.html (also expired)
http://www.patentstorm.us/patents/6617127.html
http://www.wipo.org/pctdb/en/wo.jsp?wo=2000037486
http://www.elmhurst.edu/~chm/vchembook/549aspartame.html
http://www.enotes.com/how-products-encyclopedia/aspartame
http://www.springerlink.com/index/P0141V12T42KP361.pdf (requires subscription)
http://doi.wiley.com/10.1002/bit.260320504 (ditto)
http://www.ist-world.org/ResultPublicationDetails.aspx?Resul...
There are no Google search results for "aspartyl-phenylalanine-1-ethyl ester". However, this stuff is mentioned under the name "ethyl ester of
α-L-aspartyl-L-phenylalanine" or similar in these patents, from which it appears to have been already thought of, although not yet in widespread
production:
http://www.patentstorm.us/patents/4730076/description.html
http://www.freepatentsonline.com/4730076.html
http://www.freepatentsonline.com/3678026.html
http://www.freepatentsonline.com/4293648.html
http://www.wikipatents.com/gb/1481189.html
http://www.wikipatents.com/gb/1431057.html
http://www.wipo.int/pctdb/en/wo.jsp?IA=GB2003002154&DISP...
At least one Google result also mentions the isopropyl ester, which on hydrolysis would yield isopropanol, which is oxidatively metabolized to acetone
but no further.
[Edited on 10-12-08 by JohnWW]
|
|
|
Outer
Harmless

Posts: 38
Registered: 24-11-2008
Member Is Offline
Mood: No Mood
|
|
Aspartame is 180 times sweeter than sugar, but some compounds are much more sweeter:
http://en.wikipedia.org/wiki/Lugduname
It is about 225,000 times sweeter than sucrose. So, it is more than 1000 times sweeter than Aspartame (and, probably than its Ethyl analog too).
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The proposition that aspartame releases methanol in vivo is accurate and yes that goes the usual route. However, since aspartame is so much sweeter
than sucrose, the amount ingested is small, and therefore the amount of methanol we are talking about is trivial and of no toxicological importance.
Ask yourself: what is the MW of aspartame?
Now how many typical servings of aspartame is that?
So what is the % of the MW contributed by MeOH?
So how much methanol is potentially releases from a typical serving?
If you would like some perspective, the EU limit for methanol in potable liquor is 150 mg/Kg (150 ppm).
In practice industry maintains a much lower level but that is what is allowable under law.
Methanol is not a cumulative toxin. It is rapidly metabolized and eliminated.
So let's not play sillybuggers over the "hazards" of aspartame.
Sic gorgeamus a los subjectatus nunc.
|
|
|
McLovin382
Harmless

Posts: 19
Registered: 26-9-2008
Member Is Offline
Mood: No Mood
|
|
Yes I know that the amount of methanol produced from consumption of aspartame is not really a public health hazard but it seems like using the ethyl
ester from the beginning would have made much more sense, as long as it retains some level of comparative sweetness. The ethyl group is just known to
be better for food additives as it, compared to a methyl group, is much less toxic in the first place.
The chemist who found the compound did so pretty much by accident according to wiki, and found its sweet taste when licking his finger. Why, then, if
he saw a potential for adding it to foodstuffs, wouldn't he try a less toxic ester before rushing his invention onto the market?
Not saying persay that aspartame is bad or good for you over time, just that it makes more sense to have manufactured the ethyl analog in the first
place and avoid the whole controversy altogether.
Then again people consume all kinds of baddies prolly every day without ever even realizing it. Maybe after 2,000 years of aspartame consumption we'll
evolve some kind of enzyme to protect us from methanol/formaldehyde poisoning at least a little more 
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Yes, let's fret over aspartame which might cause some problems because of the slim amounts of methanol it produces.
Better have the kiddies consume loads of sugar and give em a nice case of diabetes, much healthier!
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
The ethyl aspartame is probably not widely available because it is more expensive! It's likely to cost at least 10-fold more out of the principal that
methyl aspartame has a huge market demand and is mass produced therefore cheap per unit, whereas ethyl aspartame is not widely called for since the
methyl ester does the job and would probably be a custom syntehsis and cost far more!
I have heard about the carcinogenicity of aspartame and when I looked into it further (Oh dear I am sounding like a certain scientologist on the topic
of psychiatry!!) I found that it is the methanol from the metabolism that is the proposed carcinogen.
Well... then I figured the level of methanol produced is extremely low and quickly excreted. There are many more potent and persistent carcinogens one
will be exposed to than aspartame!
I am forever getting more suspicious of man-made chemicals entering the food chain with little longterm exposure known. Aspartame looks quite natural,
two L-amino acids and a methyl moiety, I avoid artifcial sweetners out of prinicpal but this one gets everwhere and is difficult to cut out of the
diet completely! I'd certainly be more worried about more un-natural looking artifical sweetners and colourings such as Lugduname etc which looks
totally un-natural, I'd be worried about these in my diet.
I cut out artifical sweetners, colours etc when I can. Also, recently, a little off topic, but I heard that plasticisers in plastic food packaging can
leach into food and may pose a health risk over many years of exposure, certainly in this day and age when more and more is packaged and supplied in
plastic packs and probably will continue to be, this I could see becoming a potential problem for younger generations exposed to continuous lowlevels
of plastic additives. This is yet another thing I am now trying to cut down on, if not for the environment then for my little luminal epithelia!
Any way... I (like many of us) probably expose myself to far more hazadous chemicals outside of my diet!
[Edited on 9-12-2008 by panziandi]
|
|
|
Barium
Hazard to Self
 
Posts: 85
Registered: 24-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by McLovin382
Surely someone must've thought of this before right? |
Of course a whole bunch of analogs and homologs were made and evaluated before the methyl ester was marketed. For some reason this one was chosen.
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
| Quote: | | Aspartame looks quite natural, two L-amino acids and a methyl moiety, I avoid artifcial sweetners out of prinicpal but this one gets everwhere and is
difficult to cut out of the diet completely! |
Remember, enzymes are pretty much made out amino acid chains, some of them post transitionally modified (methylated for example). There are of course
some enzymes that if ingested would seriously screw us up, so I think the train of logic does not work.
From another perspective, I can digest galactose with no problems and glucose no problems, but, if I eat LACTOSE, a seemingly innocent condensation of
the two, I will be running to the bathroom for hours thereafter
I'm not saying Aspartame is deadly; I know little about the subject, and for that matter I avoid artificial sweeteners just to play it safe.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
Methanol is a cheap industial chemical unlike ethanol. Ethanol has duty and duty on high grade ethanol is horrendous. So by using cheaper starting
materials you maximise your gross profit. I guess...
|
|
|
Outer
Harmless

Posts: 38
Registered: 24-11-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Of course a whole bunch of analogs and homologs were made and evaluated before the methyl ester was marketed. For some reason this one was chosen.
|
The question is, what was the reason?
May be, there are no reasons to prefer the "methyl" aspartame from its more "friendly" analogs.
[Edited on 10-12-2008 by Outer]
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
| Quote: |
Remember, enzymes are pretty much made out amino acid chains, some of them post transitionally modified (methylated for example). There are of course
some enzymes that if ingested would seriously screw us up, so I think the train of logic does not work. |
I am a Biochemist! What I meant was that the dipeptide of two naturally occuring amino acids is quite common and would be found naturally in your
digested food by the action of proteases on proteins etc. The methyl ester would be hydrolysed by esterases quite quickly I would imagine and the
level of methanol be low assuming normal level of intake of aspartame.
What I meant was that a dipeptide is relatively benign looking compared to some more "artifical" sweetners that are truely horrendous organic chemical
constructs taht bare no similarity to anything remotely benign... look at the structure of acesulfame, saccharin... all safe I'm sure been tested over
the years but totally un-natural looking especially the mega sweetners.
Of course enzymes are very specialised and can recognise the difference between lactose and galactose, taht is why you are lactose intolerant very
common amongst non western societies due to a mutation that causes loss of function of lactase in adults. I don't think my comment or logic was flawed
per se.
And I expect that the only reason methyl was used was cost at the time.
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by McLovin382
I don't see any reason why it would reduce the sweetness of the base compound./quote]
It most likely would change it to some degree. Could make it more or less sweet. This has a lot to do with the shape of the molecule and how it fits
into the receptor. Most drugs have analogues that are only slightly changed, not all of the analogues act slightly different however.
Also on the effects of Aspartame: http://www.ncbi.nlm.nih.gov/pubmed/17828671
I think that belittles a lot of the arguments that it is that bad.
When compared to the health affects of sugar, it might as well be considered a vitamin IMHO. |
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
| Quote: | | What I meant was that a dipeptide is relatively benign looking compared to some more "artifical" sweetners that are truely horrendous organic chemical
constructs taht bare no similarity to anything remotely benign... look at the structure of acesulfame, saccharin... all safe I'm sure been tested over
the years but totally un-natural looking especially the mega sweetners. |
Fair enough, I was just getting at the fact that no one can look at a structure and say, this is non-toxic. One can have a good idea, but without
toxicity studies there is never certainty. I am sure you know 'Natural looking' molecules can be among some of the most toxic; although, if you say
the Asp-Phe dipeptide is a common metabolite, well I can't argue with that. 
PS the ethyl ester is about 10x sweeter than sucrose while the methyl ester is about 100x sweeter.
Structure-Taste Relationships of Some Dipeptides
J. Am. Chem soc. 1969 91(10) 2684.
Attachment: dipeptide_sweetners.pdf (707kB)
This file has been downloaded 769 times
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The difference in sweetness is obviously the telling point, and not the cost of ethanol vs methanol.
There are ways round the excise tax on ethanol for applications like this. When all excise is removed, even the highest grades of ethanol (pharm,
food, cosmetic) are about $1 a liter and sold in 30,000 liter ISO tankers.
Ethanol produced from whey is available in those grades and as it is unsuitable for liquor use (except for cream liqueurs and wine coolers) due to
off-flavor, it is much cheaper than ethanol from traditional agricultural sources.
I am not talking about ethanol from petrochemical processes, which is never used for anything in the food chain.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Barium
Hazard to Self
 
Posts: 85
Registered: 24-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by panziandi
Methanol is a cheap industial chemical unlike ethanol. Ethanol has duty and duty on high grade ethanol is horrendous. |
Industrial ethanol and drinking ethanol aren't the same thing when it somes to taxing. Ethanol made from ethene doesn't differ in price much compared
to methanol. Even in the small quantities I use, 99,7% EtOH, made by fermentation of barley, is only half a Euro more expensive than MeOH per liter.
|
|
|
ItalianXKem
Hazard to Self
 
Posts: 63
Registered: 10-12-2008
Member Is Offline
Mood: No Mood
|
|
ethyl in ester group ?
it'easy , you replace methyl group with ethyl group in ester group
chemical strutture of ethyl aspartame :
NH3
.|
CH-COOH
.|
C=O
.|
NH
.|
CH-CH2-C6H5 (C6H5 is phenyl group)
.|
C=O
.|
O-CH2-CH3
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: |
I cut out artifical sweetners, colours etc when I can. Also, recently, a little off topic, but I heard that plasticisers in plastic food packaging can
leach into food and may pose a health risk over many years of exposure, certainly in this day and age when more and more is packaged and supplied in
plastic packs and probably will continue to be, this I could see becoming a potential problem for younger generations exposed to continuous lowlevels
of plastic additives. This is yet another thing I am now trying to cut down on, if not for the environment then for my little luminal epithelia!
|
But do you cut out sugar too? Sugar is also artificial in many food products which it is added to. I don't understand why people ignore the obvious
and proven danger of diabetes, while being worried about aspartame. Every time you consume a piece of fruit or drink an alcoholic beverage you ingest
more methanol than you could be exposed to from aspartame consumption.
There is a lot of pseudoscience in these "artificial food additives" debates, if you can call them debates.
Everybody who is worried about exposure to plasticizers better eliminate any smoker from his family or circle of friends. And please have someone else
fill up your car for you. You might want to drop toothpaste too, too much sodium fluoride. Etc, etc, all these things have LD50s which are orders of
magnitude higher than those of terephthalate or plasticizers.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
I didn't say I use excessive sugars in my diet I merely said I avoid the intake of chemically produced artificial sweetners whenever possible. Indeed
diabetes is a REAL issue.
I also avoid the use of plastic packaging mainly because plastic packaging is just so rediculous most of the time, and I was more raising the point
that there are people claiming plasticizers leaking into the food chain is a health issue.
I handle plenty of chemicals on a daily basis and probably these pose a greater risk. At the end of the day there are many chemicals that pose a
danger to health both acute and chronic. Take conflicting evidence on red wine being good for you. etc. It is a never ending battle between paranoid
media and gullabull public, between actual science.
I am sorry for not thinking through the cost of ethanol vs methanol properly. I assumed tax would be the issue TOGETHER with the fact that the methyl
version was in high demand. OBVIOUSLY methyl aspartame was in high demand because it was 10 x sweeter than the ethyl version!
I think the comments about toothpaste and petrol are flawed. Although yes indeed petrol contains benzene at a higher level than is permitted in
college labs in the UK! And I don't socialise with smokers and in teh UK smoking in enclosed public spaces is now finally illegal which is very nice

|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Splenda contains sucralose (trichlorosucrose, 600 X sucrose), not aspartame.
Sauron is correct. The amount of MeOH evolved from the normal amount consumed is minuscule when compared to other "benign" sources.
Increasing the chain length of the ester would, I imagine, lead to greater resistance to hydrolysis (via steric interference), but water is ~4A so the
group would need to be not only large (MW-wise), but also bulky (t-amyl, etc). This would likely introduce unacceptable solubility characteristics,
off flavors, persistance (see below) or, perhaps, unexpected toxicity.
As to toxicity, fructose (e.g. HFCS >70%) is particularly bad because it does not elicit a clear and proportional insulin response. This can result
in "insulin tolerance" which can (we suppose) evolve into type-2 diabetes. The date of onset of the obesity plague in the US is suspiciously close to
when HFCS began to replace sucrose as the sweetener of choice in cold-beverages (on account of cost).
[Edit] I know that fruit is good for you. Nature never meant for you to consume several 12 oz servings of of 20-30% fructose in a day; that would be a
S'load of fruit.
[End edit]
Sucrose is somewhat better because it hydrolyzes into one mole each of glucose and fructose. Glucose is the insulin signal, so there is an improvent
over fructose alone. I tried to eliminate excess fructose from my diet---impossible.
It is in everything (ok, almost). Frequently, it is used not only for sweetness, but as a modifier, in say, ice cream; here it serves to prevent the
formation of unwanted crystals on freezing. Gave up. Now I just drink beer (the ethanol from which would offset, but orders of magnitude, the methanol
resulting from my aspartame) and, when I can find it, Mexican Coke (the soft-drink kind).
Structure to activity ratios are particularly difficult to define when regarding what causes "sweet'. This is attributed to the wide range of
completely unrelated structures which have been found to be "sweet" (it also varies, for a given compound, between testers). The definition is still
determined (for the most part) empirically.
The aspartame moiety, however, is a common, what would you call it...sapophore (I like sucrophore better because all of the sweeteners are judged
against sucrose). Look up the newer 'tame, Neotame which adds to the aspartyl-a-amino group the neohexyl moiety. It is, IIRC 8000 times as sweet as
sucrose (http://www.neotame.com/about.asp). It still hydrolyzes rapidly via enzymic action, but the methanol so produced is again, far less. Unlike
aspartame, however, the peptide bond is less labile, so no free phenylalanine was detected in metabolic samples (or so they say); so no PKU.
I have tasted the stuff and sweet it is, but it is also persistent. The enlarged chain hydrophobizes the molecule (I don't know if this causes tighter
binding in taste receptors or not) and you mouth is "sweet" for an obnoxius length of time. Obviously, dosing and vehicle are even more important with
these super high intensity sweeteners.
My favorite is D-tagatose (marketed as "naturlose"). It's sweetness is 1:1 with sucrose (so a cake has the proper body) and it browns nicely (which
is also crucial for flavor development).
Rebaudioside A isolated from stevia extract is also quite nice, and does not have the bitter nastiness associated with the crude product.
Cheers,
O3
[Edited on 10-12-2008 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
This is hardly evidence, but, FWIIW I used to habitually drink soft drinks in vast quantity daily, when I wasn't drinking beer.
And here I am with type 2 diabetes and on a regimen of unsweetened jasmine tea (ugh) and a gram of biguanide (Glucophage) daily forever and a host of
other pills for hypertension etc.
It's no fun and Coca Cola wasn't worth it.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Again, I (nor anyone else, SFAIK) knows for sure if this is the case. But...your case seems common (which is why it is difficult to sort it,
epidemiologically). It sucks, my mom got popped (type 1, out of the blue), too. It was probably type-2 for a long time, but the Dr.s did not spot it
(despite what we regard today as obvious symptoms). Sorry, I digress.
Some of us wonder if the obesity is not a symptom, rather than the cause of the diabetic syndrome we are seeing these days. I am quite concerned,
also, that there is genetic predisposition for this.
On thread--SWAG--I wonder if the hydrolysis of the ester is not required for the binding of 'tame sweetener to occur. A decrease in rate of hydrolysis
would then explain the decrease in sweetness as the chain is lengthened. The larger, bulky group added to the back-end would serve, perhaps, to keep
it from "washing-out" hydrophobically once bound (or maybe it fits into the receptor better with that conformation?)? I'll check the literature a bit
later, when I have the chance.
Coke Zero is not at all bad if it is *cold* (Acesulfam-K and aspartame, IIRC). When warm, it tastes much more like a diet drink (blargh) with body
(which is one of the things that was lacking from Diet Coke).
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Sauron
This is hardly evidence, but, FWIIW I used to habitually drink soft drinks in vast quantity daily, when I wasn't drinking beer.
And here I am with type 2 diabetes and on a regimen of unsweetened jasmine tea (ugh) and a gram of biguanide (Glucophage) daily forever and a host of
other pills for hypertension etc.
It's no fun and Coca Cola wasn't worth it. |
Have you looked into some of the exotic supplements like the resveratrol, which its reputed qualities have been beaten to death, as well as fasting,
Every other day fasting, Caloric restriction etc?
Their is a lot of good stuff about controlling diabetes over at Imminst.org which might be of interest to you.
Also, I found this to be kind of an interesting article: http://www.foodnavigator.com/Science-Nutrition/Sugary-drinks...
Another thing to look into is the use of artificial sweeteners can stimulate appetite causing you to eat more - so I have heard. That claim however
gets a little to close to the stuff we call psychology for me to just say it makes sense and consider it as fact. 
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Fasting is a very bad idea for a diabetic. Low blood sugar is as bad or worse than high blood sugar;. The idea is to stay right around 100 and that's
what I do.
I do not use artificial sweeteners, and I restrict all carbohydrates severely. High protein, low carb, lots of fluids, plus a baby aspirin a day and
an anti-lipid pill, and a couple of antihypertensives. And of course all that biguanidine. 2 x 500 mg daily. One after breakfast and other after
dinner.
Both blood sugar and blood pressure have been well under control for 4.5 years. Life is just a bowl of cherries,
Sic gorgeamus a los subjectatus nunc.
|
|
|
myth
Harmless

Posts: 2
Registered: 10-12-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by McLovin382
...opponents say that it can cause health problems...
If that is the case, why not use an ethyl ester instead of a methyl one in the synthesis of the compound? This would cause a lot less problems and I
don't see any reason why it would reduce the sweetness of the base compound. |
Summary: It's not a chemical reason, it's political.
Before I start out I'll disclose that I've recently been converted into an "anti aspartame" from an "artificial sweetner neutral" person after I
figured out it gives me bad headaches. I'm 34 and just deduced this as I tried to eat less *real* sugar and cut calories. I can eat a little
aspartame (don't know what the threshold is...a few pieces of gum is ok, 1 packet of sweetner = hours of dull throbbing pain)...other artifical
sweetners give me no *apparent* issues...someday I'll run some experiments and figure out my threshold...
Anyway, from my reading and your question above I *believe* the reason they don't alter it is due to the enormous amount of $ and legal action it took
to get FDA approval for it. There was a lot of opposition from the FDA and other oversight people/groups and it was more of overcoming political
issues to get it approved than a logical/medical ones (spend time making it less toxic = research and development = years waiting while other
sweetners make it to market = massive $ loss for stakeholders = time to grease palms).
If you read the wikipedia article under "Discovery and approval" you may get some ideas of the politics involved...
So my guess is: Changing it to make it less toxic to the *relatively few* people that it causes problems would probably require large sums of $ to get
FDA re-approval. Better to just put a teensy little warning on products containing it and get it out the door.
Another part of this: The more competition there is amongst "artificial sweetner" products the less $ you get for the already developed and approved
product. For the owners of aspartame: Why spoil a cash generating revenue stream by "fixing" it and offering a second product...which could also
*prove* to some people that the original product did have issues... You can't stop competetion but why compete with yourself?
Note: This is all based internet research and a few books I have read that have referenced the debates/issue/politics but nothing "serious". I haven't
read "Annals of the History of Aspartame Vol. 1-10" or anything 
[Edited on 11-12-2008 by myth]
[Edited on 11-12-2008 by myth]
|
|
|
| Pages:
1
2 |