| Pages:
1
..
10
11
12
13
14
..
48 |
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Pkhow talks about K. chlorate to perchlorate; wouldn't KClO3's low solubility be a problem there.
Allowing the sol. temp to rise would dissolve more chlorate, but that would increase anode disintegration. IMO, NaClO3 would be a better starting
point for perchlorates?
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | Originally posted by Xenoid
I always detect the smell of ozone when using Pt or Pt/titanium anodes, especially when making perchlorate! |
Xenoid, are you sure it's ozone you smell, or simply something smelling like ozone.
Electrolytic production of ozone in H2SO4 solution is a well-known method, but I've never seen it mentioned in relation to chlorides or chlorates.
And, as I said, O3, being a potent oxidiser (highly thermally unstable) would be consumed fairly rapidly if it were produced at all.
Even nitric oxides, under certain conditions, can smell ozone-like.
Ozone is, of course, detectable in air in very small concentrations, so it's just possible a few PPM could be released over a period.
'Way too small to be of any, but academic interest.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
About Ozone coming from Perchlorate cell, the following is from US Patent (attached).
.....................
However, as the presence of unconverted chlorate in perchlorate imparts very serious and undesirable characteristics to the completed product, it is
preferable to operate the cell to the lowest concentration of chlorate that is economically feasible. The economic limit is reached when a
concentration of about 1 to 5 g/l chlorate is reached. This is due to the fact that the high current density of 450 amperes per square foot
(480mA/cm^2) produces excessive quantities of ozone by reason of electrolysis of the aqueous electrolyte, thus lowering the cell efficiency to
unacceptable levels.
The perchlorate cell solution at the end of the conversion is, ...........................
Dann2
Attachment: US Patent No_ 2,392,769.htm (7kB)
This file has been downloaded 1222 times
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Thanks indeed for that, dann2, I stand corrected.
It's hardly likely the patentee mistook ClO2 for O3?
It's quite interesting, actually, as ozone will oxidise NO2 to N2O5.
Perchlorates from one container---nitric acid anhydride from another (or am I losing it?).
That stated current density, though, is highly unlikely to be seen in a home set-up.
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by hissingnoise
That stated current density, though, is highly unlikely to be seen in a home set-up. |
Yes, true! But I assume on sharp edges, points, etc. one can get a "localised" high current density, much in the same way as with high voltages
ionising air at needle points.
I guess it's the reason the "ozone" anode is only an edge of platinum 0.1 mm thick, exposed at a glass surface - very high current density.
Also, we're probably not talking about large quantities of ozone here, the nose is quite sensitive to this gas after all.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Thanks for the O3 clarification. The odd thing was, it seemed to be produced immediately, indicating "poor efficiency", yet the rig went on to make a
decent batch of perchlorate.
I hope to put to rest the "MMO makes/cannot make perchlorate" question with this simple test cell:

It's 350 grams of cell chlorate dissolved in 2.5 liters of water. Because the setup is small, it's running at only 15 amps, so it'll be a while
before any conclusions can be made. There are certainly trace amounts of chloride, but it is a predominantly clean chlorate solution to start with.
I've been gathering chemicals to plate one of these anodes, and I hope to give it a shot this weekend. I'm going to try a 30 RPM gearmotor to spin
the anode, hopefully creating an even and bubble-free plating.
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
Swede: How will you arrange the plating cathode(s) so that the anode will be plated evenly? Not trying to put the kybosh on your idea, but
traditionally spinning is combined with round anodes.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by tentacles
Swede: How will you arrange the plating cathode(s) so that the anode will be plated evenly? Not trying to put the kybosh on your idea, but
traditionally spinning is combined with round anodes. |
If a flat anode and a rod cathode are sufficiently far
apart, spinning a flat plate given a close-to-flat average current distribution. The worst case in terms of anisotropy is the flat plate lying in the
same plane as the rod. As the distance between them increases, the current density between the close edge and the far edge equalizes.
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
Hello Swede,
One coating I've always been curious about is PdO (over MMO). Several patents imply that PdO makes for a more efficient MMO coating than RuO2 or IrO2
(for chlorine evolution at least), and one paper I seem to remember (regarding electrodes for waste-water treatment or something), found PdO to be
superior.
IIRC one of the examples in one of Beer's patents uses anodic electrodeposition of PdO from a dilute PdCl2 solution. (Of course Beer's plating
solution also contained TiCl3 I believe, but if you are plating over pre-existing MMO, you probably don't need to co-deposit TiO2 with the Pd).
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
Perchlorate as a Contaminant in Chlorate Production
Being that MMO anodes are used in the chlorate industry, I thought that if they are capable of making perchlorate, then there may be some patents on
reducing/eliminating perchlorate from the chlorate electrosynthesis process.
IOW maybe something can be learned about which type of MMO anodes would be best for perchlorate production if we can find out how the chlorate
industry minimizes perchlorate contamination.
As it turns out, I found a few patents on the subject (which implies that MMO anodes can/do make perchlorate, and that this may be a potential problem
in chlorate production), but the patents unfortunately didn't go into any detail on anode design.
According to US5063041:
"A problem in chlorate processes is the formation of an undesirable amount of perchlorate which is continuously enriched in the cyclic process. The
formation of perchlorate is associated with a poor function of the anodes and can be overcome partly through a careful control of the process
conditions and of the selectivity of the anodes. It is possible that up to 0.5 g sodium perchlorate per kg sodium chlorate is formed, despite such
control. This is equivalent to an increase in the concentration per year in the order of 5 to 10 g sodium perchlorate/l in a chlorate plant with
normal power density."
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
On anode spinning - my experience with plating has been limited to decorative plating. With nickel, the spinning produced mild agitation, prevented
the bubble formation that you'd find in a more stagnant plating tank, and produced a very even plating. Is there a problem with this in regards to
PbO2 deposition?
With a top down view, using a single plate cathode, and an MMO-mesh target anode, it would appear that the current density over the entire surface of
the anode would be a nice average - the exact middle (axis of rotation) of the anode would receive a relatively constant current, and as you progress
to points away from the axis, the current density would be sinusoidal, with the amplitude increasing as you reach the edge. But wouldn't the average
current each point "sees" be the same?
I am not hard-set on rotating the anode, I'm just going by what my limited experience with plating tells me works well.
jpsmith, is the PdO coating targeted at chlorate production, or perchlorate? I couldn't tell from your post if the PdO coating is designed to
REPLACE, or simply COAT, an existing MMO surface?
On the MMO --> perc rig: No sign of perc yet, but it's early. No visible flecks of black MMO coating either, which is a good sign. This is just
for fun, to finally put to rest the "DOES it, or DOESN'T it" question.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Swede
With a top down view, using a single plate cathode, and an MMO-mesh target anode, it would appear that the current density over the entire surface of
the anode would be a nice average - the exact middle (axis of rotation) of the anode would receive a relatively constant current, and as you progress
to points away from the axis, the current density would be sinusoidal, with the amplitude increasing as you reach the edge. But wouldn't the average
current each point "sees" be the same? |
The current average isn't identical, as it might seem. For a first
approximation, think of each point on the rotating anode as grounding through a resistor proportional to its distance to the cathode. All these paths
pass current in parallel. If the anode and cathode are too close, the closest edge will hog all the current. Current density is more even when the
values of those parallel paths are closer, which is what happens when they're adequate far apart.
It's even worse than this at the next level of approximation, to trace out the equipotential surfaces and to estimate the electric field within the
electrolyte. The fields from the far edge enough curve out away from a straight line path significantly, causing even greater resistance. Fortunately,
the same solution (move them away from each other) addresses the problem.
If they're too close, the bulk of the plating will be on the edges, leading to something of a barbell cross-section.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Hmm, I knew it would never be as simple as I thought! Would there be ANY advantage to rotation, or would it be an unnecessary complexity? Would
stirring, then, be a superior method to create as even a coating as possible?
I finally got around to weighing the yield and calculating the efficiency from my new cell's first run. Yield was better than I thought. The dried
crystals weighed in at 4,229 grams, or 34.50 moles of potassium chlorate. But the 4,229 grams is a dry yield... significant quantities of potassium
chlorate, converted from chloride, remain in solution. The cell holds 25 liters. 14 of those at the start were used liquor, presaturated with
chlorate, plus added chloride. 11 liters was pure KCl solution, thus, the system had to saturate those 11 liters with chlorate before even the first
crystal would form. 11 liters at 20C should hold 880 grams. Thus the final quantity is closer to 5,109 grams, or 41.67 moles.
The system required 7580 amp-hours to produce this quantity.
7680 / 41.67 = 184.3 AH/mole
100% efficiency is 160.8 AH/mole; my efficiency is therefore 87.2%.
This seems a bit high. This calculation is the best I could do, and the "bonus" 880 grams is just a guess. With this sort of system, there is always
chlorate produced but never crystallized. Still, my best yield in a non pH-controlled environment was 62%, and I am very happy with an efficiency in
the 80's. A fairly hot cell, good pH control = mounds of oxidizers! 
Sorry I keep editing this post. Being tired of having to look up all the assorted numbers for calculating efficiency, I came up with this formula for
dry yield only:
For Potassium Chlorate Production:
W = Weight of yield, in grams
E = Efficiency
AH = Ampere-Hours used
E = (131.32 * W) / AH
[Edited on 18-11-2008 by Swede]
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Swede
Hmm, I knew it would never be as simple as I thought! Would there be ANY advantage to rotation, or would it be an unnecessary complexity? Would
stirring, then, be a superior method to create as even a coating as possible? |
Rotating seems fine to me.
You'll incur greater resistive losses and maybe plate on a little heavy at the edges in order to get adequate coating in the center. All well within
the imperfections of small scale.
If you want to stir instead and use fixed electrodes, you'll need to change the electrode geometry. Use a pair of plate cathodes as close as feasible
to the anode and make them extend significantly past the edges of the anode, say, a cathode width three times that of the anode. That'll give you a
pretty uniform electric field and even plating.
[Edit: Saying "stirring" when you mean "rotating" can be pretty confusing.]
[Edited on 18-11-2008 by watson.fawkes]
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
Swede I was thinking that the PdO would go over the existing MMO coating, just like what you apparently plan to do with the lead dioxide.
According to a patent I happened to see, supposedly Pd has the highest "selectivity for chlorine" out of all the noble metals. I suppose that suggests
PdO for chlorate production...perchlorate usefulness remains to be seen.
As far as putting Lead Dioxide over MMO, I wonder, do you really need to worry about pinholes? Being that the MMO would apparently be such a robust
intermediate layer, maybe it wouldn't be worth the hassle.
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
The only problem I can see with rotation is the uneven current density - you might have alpha PbO plating at the edges and beta in the center of the
anode, or some sort of mixed phase variation.
jp: The problem with noble metal oxides, is that noble metals are quite pricy.. LD has the benefit of being cheap and rather easily made.. It's
nowhere near $25-50/g. Especially when you consider the cost of noble metal salts. If you're going to go to the expense (and fiduciary risk)
of putting noble metal coatings over MMO, you might as well just buy a Pt plated anode and be done with it.
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
Hello Tentacles,
If MMO anodes were not such a great value, they wouldn't be used so much in industry.
MMO anodes can be bought on Ebay for something like $39.00. That's because even though the noble metals are relatively expensive, unlike PbO2, only
thin coatings are required, i.e., only a few microns or so. (And, used properly, supposedly they are much more resistant to erosion than Pt).
PdCl2 can be bought on Ebay for about $20 per gram last time I looked. A few grams would be enough to make a liter of plating solution and that should
be enough to coat a few anodes I would think.
Even if PdO over MMO doesn't make perchlorate, maybe it would allow a "user repairable" MMO anode without the hassle and toxicity of PbO2.
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
jp: Why not just make RuO2 based anodes like the industry chlorate anodes (although those might not be straight TiOx/RuO2)? I mean, if you're going to
make your own MMO, why not just do it? And, as you stated, MMO are cheap and readily available, so why repair? I'm not trying to stop the research
here, just trying to point out that it might not be feasable or even the best course of action.. I think pool chlorinator anodes should be easy to get
just about anywhere, and are innocuously shipped in more remote regions etc.
Anyone is certainly welcome to try, but my opinion is that it's probably not as easy as it would seem.. Look at 12AX7's Pt plating experiments, etc.
That doctoral thesis had a MMO anode dip n bake procedure that is apparently what is used to make industrial chlorate MMO. I found it quite
interesting, as well, that a thicker coat isn't necessarily longer lasting.
Again, I'm not saying that it's not possible to make a good, reliable perchlorate anode based on noble oxides over MMO (or what-have-you), I'm saying
that it's probably not economically feasable, particularly compared to the ease and availability of PbO2 plating.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Spinning the MMO should definitely help to eliminate bubbles/pits. Since you have a motor (I presume) it will not be too much trouble. You will need a
brush for the current (more hassle).
If you have a reasonably sensible arrangement of Cathodes the set up will be fine IMO. The edges of the MMO will come close to the Cathodes as it
sweeps around and you may get heavier plating when compared to the center part of Anode.
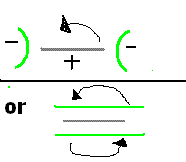
Perhaps an arrangement like below may help to even out the average current distribution on the rotating Anode,
or perhaps spin the two Cathodes and the Anode as a single unit (two brushes now needed, more hassle).
When using Graphite as a substrate you always had to round off all sharp edges from the Graphite becasue if you did not, large amounts of LD would
deposit on the corners/edges. (Ugly anodes, and you don't want one of those now, do you!!)
Tentacles did not seem to have this problem though but it will not start to show up much untill you put on a thickish coating.
If you look at the close up of the mesh you can start to see a thicker amount of LD going on at the top of each hole in the mesh. Nothing you can do
about it and it does not really matter anyways.
From elsewhere:
________________________________
Another parameter that is mentioned regarding plating baths is 'throwing power'. The addition of Nitric acid is said to improve the throwing power (an
advantage).
Throwing power refers to the ability of the solution to plate into nooks and crannies and around corners that are not in a direct line of sight of the
cathode. To put this another way, it is the ability of the electrolyte to even-out the current distribution on all areas of the anode given a certain
tank and electrode set up. There is a good explanation of throwing power in "Treatise on Electrochemistry" , G. Kortum, (Elsevier publishing Co.).
It is related to the conductivity of the solution and Nitric acid increases this. The current on the anode is more evenly distributed as a result of
the greater conductivity. The current distribution is also a function of cell geometry, spacing and arrangement of the anode and cathode. The cathode
should surround the anode being plated and there should not be any sharp corners (this increases the current density and increases plating on that
area) or indents (less plating) on the anode.
_____________________________________
Dann2
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: |
jp: Why not just make RuO2 based anodes like the industry chlorate anodes (although those might not be straight TiOx/RuO2)? I mean, if you're going to
make your own MMO, why not just do it?
|
I'm not advocating anyone making their own "MMO" per se. Why do that? As far as making chlorate is concerned, you can buy a half-decent pool
chlorinator (complete with Ti cathode and associated hardware) for $100 to $150 that will probably last a few years and take a lot of abuse.
Sorry if I wasn't clear but what I'm suggesting is using a commercial MMO coated anode as a substrate for a PdO outer layer (obviously as an
*experiment* at this point), which may possibly make perchlorate at an acceptable efficiency. If it doesn't, then as a worst case (being that you
already have the MMO anode and the plating solution), you may end up with a "user repairable" anode albeit only for chlorate.
| Quote: |
And, as you stated, MMO are cheap and readily available, so why repair?
|
As per the above, because you can repair.
| Quote: |
I'm not trying to stop the research here, just trying to point out that it might not be feasable or even the best course of action.. I think pool
chlorinator anodes should be easy to get just about anywhere, and are innocuously shipped in more remote regions etc.
Anyone is certainly welcome to try, but my opinion is that it's probably not as easy as it would seem.. Look at 12AX7's Pt plating experiments, etc.
That doctoral thesis had a MMO anode dip n bake procedure that is apparently what is used to make industrial chlorate MMO. I found it quite
interesting, as well, that a thicker coat isn't necessarily longer lasting.
Again, I'm not saying that it's not possible to make a good, reliable perchlorate anode based on noble oxides over MMO (or what-have-you), I'm saying
that it's probably not economically feasable, particularly compared to the ease and availability of PbO2 plating.
|
Well this is all presumptuous since neither PbO2 nor PdO over MMO have been tried yet, AFAIK, but if buying a few grams of PdCl2 at about $20 per gram
is a significant economic hardship, and if you can get everything you need to do PbO2 plating for free or at a negligible cost, and you don't mind the
toxicity and the mess, then go for it, I suppose.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by dann2
Another parameter that is mentioned regarding plating baths is 'throwing power'. The addition of Nitric acid is said to improve the throwing power (an
advantage). [...] It is related to the conductivity of the solution and Nitric acid increases this. The current on the anode is more evenly
distributed as a result of the greater conductivity. |
This is another way of getting more even current
densities, for essentially the same mathematical reason. The multiple current paths (properly speaking, the integral curves of the electric field)
have resistances closer to each other, so current density evens out. In this case higher conductivity changes the shape of the electric field. You can
use this technique in conjunction with manipulating the geometry by increasing the resistance.
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by jpsmith123
Sorry if I wasn't clear but what I'm suggesting is using a commercial MMO coated anode as a substrate for a PdO outer layer (obviously as an
*experiment* at this point), which may possibly make perchlorate at an acceptable efficiency. If it doesn't, then as a worst case (being that you
already have the MMO anode and the plating solution), you may end up with a "user repairable" anode albeit only for chlorate.
Well this is all presumptuous since neither PbO2 nor PdO over MMO have been tried yet, AFAIK, but if buying a few grams of PdCl2 at about $20 per gram
is a significant economic hardship, and if you can get everything you need to do PbO2 plating for free or at a negligible cost, and you don't mind the
toxicity and the mess, then go for it, I suppose. |
Sorry, I was a bit confused there.. Pd might indeed work well, but I suspect you'll need more than a couple grams.. Probably more like saturated
solution, so that you don't deplete all the Pd ion in the plating operation.
I'm assuming the PdCl plating bath would be somewhat dilute HCl? Is there some information on the process, I might be willing to give it a try. I
wonder if you can add some TiCl3, if that would electroplate a mixed oxide film.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Current density follows the same pattern regardless of resistivity. There must be a surface effect in action (ligand, electric layer, etc.).
Tim
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
PdCl2 has quite poor solubility, so I automatically assume an acidified (therefore complexed) palladium (II) solution. Also, this solution is somewhat
sensitive to oxidation.
For any who mess with platinising titanium, I suggest diamminedinitrite platinum (II) solution as it actually works. In retrospect, I wish I had sent
that to Tim for he would have had success with it. This complex is easily made from K2PtCl4.
I have ruthenium metal for sale. I also have platinum, palladium, rhodium and some of their salts (halides, oxides, sulfides, etc) available to you
lot here.
Of course the precious metals salts can be free of charge if you'd but share your chlorate and perchlorate with me :-)
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by 12AX7
Current density follows the same pattern regardless of resistivity. There must be a surface effect in action (ligand, electric layer, etc.).
|
The polarization resistance (constant at low current, drops off at high) of the electrodes stays the same,
but the bulk electrolyte resistance drops. Hence the total resistances along each conduction path grow closer to each other.
|
|
|
| Pages:
1
..
10
11
12
13
14
..
48 |