| Pages:
1
..
18
19
20
21
22
..
45 |
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
It's one of those mystery-catalyst things.
Regarding the pruported reaction of HCl gas and P2O5: It can't work. Here is why.
If you succeed in forming POCl3 from P2O5 and dry HCl, you split off H2O in the reaction.
The water can hydrolyze any POCl3 formed, but let's assume that you are lucky and the water reacts selectively with more P2O5 present instead. This
will form H3PO4 so you now have in your pot: P2O5, POCl3 and H3PO4. H3PO4 and P2O5 together form polyphosphoric acid and if you think POCl3 will
peacefully coexist with any of the above I suggest you read a little more fundamental Phosphorus chemistry because it won't.
Let's look at some stoichiometry:
6 HCl + P2O5 -> 2 POCl3 + 3 H2O
so we need excess P2O5 to remove the water
P2O5 + 3 H2O -> 2 H3PO4
So 1 mol excess P2O5 will do that. But now we have 2 mols H3PO4 in our pot along with 2 mols POCl3
I think it is naive to assume that the water formed from reaction of HCl gas and P2O5 will not destroy any or all POCl3 formed.
So this reaction is a muddle.
Can POCl3 be distilled off as it forms? Maybe but I doubt it.
[Edited on 29-10-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
In Gmelin you can find references for the preparation of POCl3 from P2O5 by using HCl, NaCl, CaCl2 and several other chlorides. HCl reacts slowly at
room temperature and it takes several days to reach the equilibrium between polyphosphoric acid and POCl3. Alkali chlorides require reactive
distillation of POCl3 out of the mixture as it forms and the reaction proceeds only at >200°C for most chlorides. If I remember correctly only
VOCl3 is mentioned of forming POCl3 from P2O5 at room temperature in chloroform as solvent. It is several years since I checked Gmelin for these
reactions so I might have remembered wrong about some details and besides I never went to read the original papers (I only have access to Gmelin at a
library that is not close enough to just go and check again).
Interestingly, I could find nothing about the reaction of P2O5 with Lewis acid type chlorides such as FeCl3, ZnCl3 or AlCl3 which I would imagine
should react at much lower temperatures than alkali chlorides (particularly FeCl3 given the favourable reaction thermodynamics). Apparently there was
not enough interest in these reactions for the inorganic chemists to check.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I've previously posted the reaction between NaCl and P2O5 which is done under autogenous pressure in an autoclave at 400-500 C. This was reported in
JACS in 1940 by Tarbutton, I posted the paper which is mostly concerned with the analogous reaction with fluorides to form POF3 gas.
The byproducts are alkili phosphates.
A little PCl3 also forms, probably, according to Tarbutton, from Fe catalysis from vessel wall. This is up to 10% of the POCl3. HCl forms in
proportion to any moisture in the NaCl.
It is an open question as to whether Tarbutton's hypothesis could be exploited to up the Fe catalysis and obtain a higher proportion of PCl3, or even
exclusively PCl3, which would make me very happy as there is no good way to prepare PCl3, not even from red P or from PCl5. Oh, there are procedures
but ask garage chemist who has tried them and he will support my generalization.
Whereas there are lots of ways to make POCl3.
The particular prep advanced upthread did not involve a closed system, just bubbling dry HCl into P2O5 (I assume with exclusion of atmosphere) and
without any discussion of reaction time or stoichiometry. I am damned sure POCl3 will react with H3PO4, PPA or P2O5 to ptoduce some higher oxo acid or
anhydride. PPA as you know is not a simple H3PO4/P2O5 system but a complex mixture. So I have serious doubts about the preparative usefulness of P2O5
(excess) + dry HCl.
In the rxn of P2O5 with NaCl (and other chlorides) the temperature required happens to coincide with the vapor phase of P2O5 and also the transitional
phase in its structure. Tarbutton makes no mention, but of you look at the lit. on reaction of P2O5 with anisole under similar consitions this aspect
is discussed in detail. (The product when P2S5 is used is Lawsson's Reagent.)
None of that is happening in this low temp reaction with HCl at atmospheric pressure.
[Edited on 30-10-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Further to the above, Tarbutton's reaction was conducted on the basis of the stoichiometry:
3 NaCl + P2O5 -> POCl3 + Na3PO4
in an iron or stainless steel bomb. The reaction with NaCl initiated at 250 C while that with CaCl2 required 400 C. The product mix was 75-90% POCl3,
10-25% PCl3 which the author speculated was from reduction of POCl3 by the vessel wall. The HCl in small amount was proportional to moisture in the
reactants.
[Edited on 29-10-2008 by Sauron]
Attachment: tarbutton.pdf (807kB)
This file has been downloaded 1566 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
That's sounds to good to be true. What is the reaction time?
If it's not too long, this may be as well a good route to acetic anhydride, hard to get for many of us (I'm blessed with a liter of the stuff).
In the acetic anhydride thread, I saw from anhydrous sodium acetate, acetyl chloride can be prepared via POCl3. Acetyl chloride then react with the
exess sodium acetate to form acetic anhydride. This process was first not so preferred, due to the difficulty obtaining POCl3. But if it's this easily
prepared and cheap (phosphorus pentoxide is quite cheap, at least it is at Acros/Baker, where I can buy). NaCl? Well i don;t have to tell.
I read in the PDF that only POCl3 is formed in a glass vessel exclusively.
So heating a mix of NaCl, anhydrous sodium acetate and phosphorus pentoxide will give acetic anhydride?
However it would be important that the phosphorus pentoxide is dry, or metaphosphoric acid and pyrophosphoric acids might form, wich severely attack
glass at those temperatures. AFAIK P4O10 does not, but I'm not sure.
If anyone wants to try this, please post results. I currently do not have P4O10, and it will take at least a month before I get it. I will buy it by
the 500g (approx 15 EUR) or by the Kg (Acros, 26 EUR). Damn cheap chemical...
However, opening the container will significantly reduce quality right? So it might be be better to go for 2x 250g , a total of 20 EUR.
[Edited on 29-10-2008 by Jor]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
In my opinion there are a dozen better ways to make Ac2O than using POCl3, it is precious stuff. I listed these upthread.
I'm not sure what the reaction time is. It sounds like, with NaCl, the volatile products start distilling off as reaction commences c. 250 C. In a 1 L
autoclave with valve open to a delivery condenser, how long will a mol of POCl3 take to distill? You won't get much more than 3 mols NaCl and 1 mol
P2O5 into the working capacity (650 ml) of that vessel. Maybe 1.5 mols, probably not 2.
If 10 to 25% of the POCl3 produced is reduced to PCl3 as Tarbutton says, then I would fractionate these apart, the PCl3 is even more precious. I'd
love to be able to reduce it all to PCl3.
Oxidizing PCl3 to POCl3 is a known process, reagent is KClO3. But it's the reduction that is more interesting.
I DO NOT recommend trying to combine the reactions, if you insist on wasting POCl3 to make Ac2O then preform the POCl3 and then react with anhydrous
NaOAc. The PCl3 if any will not hinder anything as PCl3 also reacts with NaOAc to make Ac2O. But there are better ways! Benzoyl chloride. Phthaloyl
chloride. TCT. If you can buy from Acros, you can buy cyanuric chloride and it is cheap.
The P2O5 does need to be dry or you just make HCl. Open it in a dry box (glove box) or at least an atmos-bag. P2O5 is flocculent and a mess to
transfer. I buy the 250 g bottles and use them in one go.
Here is first pahe of the 1954 JACS paper discussing the three crystalline modifications of P4O10, their structures, the transition temperatures and
associated vapor pressures. The temperature ranges are the same as for Tarbutton's reaction and I say this is no coincidence. In this transition some
P-O bonds are broken and clearly the forms at these temperatures are more reactive. See also the reference to another JACS paper by Hill and coworkers
cited on this page.
[Edited on 30-10-2008 by Sauron]
Attachment: f_ja01633a035.pdf (132kB)
This file has been downloaded 1773 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Yes Sauron I understand POCl3 is precious stuff, but I do not think it is if this method really works. P4O10 is cheap, and not so hard to get for most
of us. (not regulated)
So if POCl3 will be be easy to make from available chemicals, it is not that 'precious' and it can be used to make Ac2O.
It's quite interestng for me, POCl3 is impossible or me to get, just as SOCl2, PCl5 and PCl3. That is because is list 3 CWC. I can however buy about
any chemical at Acros/Fisher/Merck/Baker/Aldrich, but not CWC or some others. Red P, I can buy, so if I need it I can make PCl5 (I have about 250g red
P).
Im not interested in the Ac2O, I have a liter here, wich I hardly use.
I know TCT is very useful and cheap. if I want I can buy it from Acros. But I dont' want it, as it is classified as 'very toxic by inhalation'. Is it
that bad? Is it like NO2/Cl2 or like fosgene/AsH3/H2Se?
Fortunately I am almost finished building a hood.
I do not have a glove box. I want to make a dry box sometime. A box where I put the chem in, then dry it for about 2 hours with KOH or something like
that, and then open container with inbuilt gloves. Future project.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The glove/box/dry box is one issue, you can ger around it by using a cheap and disposable Atmos-bag assuming you have a cylinder of N2 or Argon.
You will also find that glass will not take contact with this reaction mix at these temperatures, so you will go through a few flasks but that is not
a big deal. Really better to do this in stainless steel autoclave and if you haven't got one, it's a major expense.
The NaCl ought to be well dessicated. Baked out in a drying oven or heated in a crucible. I don't think it needs to be fused, just drive off any
moisture.
Another very good way to make POCl3 is to buy red P, chlorinate it to PCl5, and use that to prepare oxalyl chloride from anhydrous oxalic acid. The
byproduct is POCl3. But if you can't get red P this is a non-starter. It is actually a better way to make POCl3 than it is to make oxalyl chloride.
That's because you need a lot of PCl5 (3 mols plus escess) to treat one mol oxalic acid (anhydrous) and even so yield is <50%. You get 3 mols
ZPOCl3, though.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Here’s what Gmelin mentions
| Quote: | Originally posted by Nicodem
In Gmelin you can find references for the preparation of POCl3 from P2O5 by using HCl, NaCl, CaCl2 and several other chlorides. HCl reacts slowly at
room temperature and it takes several days to reach the equilibrium between polyphosphoric acid and POCl3. Alkali chlorides require reactive
distillation of POCl3 out of the mixture as it forms and the reaction proceeds only at >200°C for most chlorides. If I remember correctly only
VOCl3 is mentioned of forming POCl3 from P2O5 at room temperature in chloroform as solvent. It is several years since I checked Gmelin for these
reactions so I might have remembered wrong about some details and besides I never went to read the original papers (I only have access to Gmelin at a
library that is not close enough to just go and check again).
Interestingly, I could find nothing about the reaction of P2O5 with Lewis acid type chlorides such as FeCl3, ZnCl3 or AlCl3 which I would imagine
should react at much lower temperatures than alkali chlorides (particularly FeCl3 given the favourable reaction thermodynamics). Apparently there was
not enough interest in these reactions for the inorganic chemists to check. |
The formation by NaCl and P2O5 was long ago described by Lautemann in Lieb. Ann. 113 [1860] 240, but also Kolbe and Lautemann in Liebigs Ann. Chem.
147 [1868] 355/66, 361. The preparation through P2O5 with CaCl2 and NaCl in a Fe- or steel vessel , through the reduction of POCl3 with the Fe of
the vessel some PCl3 forms, the reaction starts at 250ºC with NaCl, and 400ºC with CaCl2, which is from the Tarbutton already mentioned in this
thread (JACS. 63 [1941] 1782/9, 1785). Gaseous HCl is absorbed at first slowly from P2O5, then quickly absorbed to a maximum and then gets absorbed
more slowly again, likely according to the equation: 2 P2O5 + 3 HCl = POCl3 + 3 HPO3 (J. chem. Soc. 53 [1888] 756). Another way from P2O5 is by
heating it with CCl4 to 200 to 210ºC in the ratio of: P2O5 + 2 CCl4 = COCl2 + CO2 + 2 POCl3, or 2 P2O5 + 3 CCl4 = 4 POCl3 + 3 CO2 (Ber. 5 [1872] 30;
Z. Chem. [2] 7 [1871] 615). Clearly aim for the latter reaction where phosgene shouldn’t be formed. Several other methods are covered, there is
indeed no mention of Lewis acid chlorides directly in a synthesis.
Sources: Gmelin, Syst. No. 22, Na, p. 329; and Syst. No. 6, Cl, p. 130; Syst. No. 16, P, Tl. C, p. 458-462.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Thanks. Tarbutton's paper was primarily about the reaction of P2O5 with fluoride salts and the preperation and characterization of PF3, POF3 and some
mixed chlorofluorides. The NaCl and CaCl2 reactions were a passing comparison, as were some reactions with rock phosphates. He did cite some
references, but nothing as old as the Gmelin entries, and unfortunately not much of it accesible to me.
It is nice to have confirmation.
The CCl4 reaction is interesting. NaCl is a lot easier to come by though in these parts. I can get that Berichte paper from BnF, and the JChemSoc
paper from RSC.
Thanks again!
Because now we can put this turkey to rest.
The reaction between HCl gas and P2O5 at normal temp and 1 atm requires almost 600 hours. 25 days, 3.5 WEEKS. The bulk of the uptake occured in first
5 days but absorption was still proceeding when they ceased measurements at 25 days.
The authors did not measure anything at elevated conditions, because they were just trying to clarify an anomaly in drying HCl gas stream over P2O5.
Now, perhaps this can proceed at a less leisurely rate at 1900 psig (the limit of my autoclave) and 325 C but we do not know that, so I must ask you
in all honesty, is that what you would regard as a preparatively useful reaction?
It always pays to read the actual papers because reviews like Gmelin often omit insignificant details, like whether a reaction is actually good for
anything. Like in this case, answer is "No."
[Edited on 2-11-2008 by Sauron]
Attachment: ct8885300755.pdf (409kB)
This file has been downloaded 1287 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The CCl4 reaction with P2O5 at 200-210 C takes place over 48 hours, and proceeds at an 80% yield (phosgene basis) in the case of the first
stoichiometry.
This is not so worthless if one has a source for CCl4.
I agree that the phosgene-free second stoichiometry is to be preferred.
Here is the page 30, it is just a couple of paragraphs describing a report by a Herr Gustavson. Incidentally I got this from BnF because Wiley is
missing Issue 1 of Volume 5, Berichte, so I could not get a proper citation there.
[Edited on 2-11-2008 by Sauron]
Attachment: 30.pdf (57kB)
This file has been downloaded 1430 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Thanks for finding the papers, in the Ber. they are also saying that only traces of COCl2 form in the 2 mol P2O5 and 3 mol CCl4 reaction. The original
seems be the paper by G.Gustavson in the Ztschr. Chem. citation. They are also saying POCl3 and excess P2O5 react to form a thick, transparent mass
that is left over after the POCl3 was distilled off. Further I’ve found that this mass is metaphosphoryl chloride: which forms when P2O5 and
oxychloride are heated in the right ratio to 200º during 36hrs: P2O5 + POCl3 = 3 PO2Cl (Gustavson, Ber. 4, [1871] 853) and speculates it might be
mixed with a polymer. Huntly (J. chem. Soc. 59 [1891] 202-8) says the reaction is more complicated and forms additionally P2O3Cl4 and P7O15Cl5. The
reason why Gmelin cuts short on details is because this information didn't appear under the main preparation entries, which stick out like sore
thumbs. Here is the files, they are clearest at about 65 to 75% zoom.
[Edited on 2-11-2008 by Formatik]
Attachment: Gmelin-POCl3-PCl3-PCl5.pdf (2.2MB)
This file has been downloaded 1292 times
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Thanks, I do appreciate your kindness.
The full cirarion for the above paper:
Vorläufige Mittheilung über die Reaction des Phosphoroxychlorids auf Phosphorsäureanhydrid
G. Gustavson
Bar., 4, 853 (1871)
DOI: 10.1002/cber.187100402104
I will post it shortly.
Meanwhile here is the 1891 Huntly paper from J.Chem.Soc.
[Edited on 3-11-2008 by Sauron]
Attachment: ct8915900202.pdf (465kB)
This file has been downloaded 1363 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
G.Gustavson:
Regarding his report of the reactions of CCl4 and P2O5 at 200-210 C for 48 hrs, I would be interested in the mechanism of this reaction. A thermally
initiated free radical mechanism seems likely, one involving perhaps the initial formation of the ubiquitous (with CCl4) trichloromethyl radical and
a chlorine radical. If this is a FR reaction then perhaps temperature or time can be reduced by UV photolysis, or peroxide initiation.
A further question is whether a similar reaction works with CHCl3.
P2O5 + 2 CHCl3 -> 2 POCl3 + H2O + 2CO perhaps?
The short note attached concerns his study of the reaction of P2O5 and POCl3 to give metaphosphoryl chloride at 200-120 C/36 hours.
[Edited on 3-11-2008 by Sauron]
Attachment: 853.pdf (45kB)
This file has been downloaded 1399 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Back to the uptake of dry HCl by P2O5:
I should not have been so dismissive of this. I think that run as a pressure reaction at elevated temperature this is probably very practical and
certainly as cheap as NaCl or cheaper. Most members simply do not have any pressure equipment to speak of.
A Parr bottle will at best afford 6 bar and 90 C. That is pushing things a little.
What Ace sells as glass pressure reactors are 40-50 psi (3 bar)
A Parr autoclave, of SS and normal design 1900 psi and 325 C.
The above are listed in increasing order of cost and decreasing likelihood of being in a home lab.
I have the first and third. 500 ml Parr heated shaker and 1 L Parr stirred heated pressure reafctor.
I do not presently have bottled HCl gas. The required stainless steel regulator for a full sized cyclinder is pricey, but sooner or later I will have
to get one. I already have ordered 5 SS diaphragm valves for lecture bottles so I can buy a few LBs from Aldrich, and have them filled locally. Enough
HCl to see what conditions work most efficiently.
----------
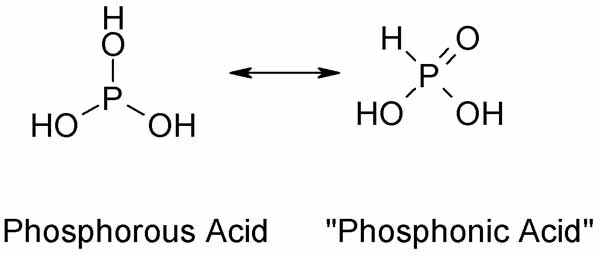
A complication when trying an unknown reaction with H3PO3 is that it is a tautomeric compound. Usually it exists as "phosphonic acid" HP(=O)(OH)2in
pentavalent state. But its triesters and trihalides are the tervalent form reflecting the parent structure of phosphorous acid P(OH)3.
Its anhydride P4O6 (aka P2O3, phosphorus (III) oxide or trioxide, or tetraphosphorus hexaoxide, reacts very differently with water than does P4O10.
The latter merely hisses when thrown into water and forms H3PO4 like a well behaved anyhydride.
P4O6 added to hot water decomposes violently to phosphine, H3PO4 and red P. In cold water it is slightly less spectacular.
So my hypothesis about its reaction with oxalyl chloride are rather speculative. However as P4O6 is "unobtainium" I won't be finding out. I'll be
reacting the oxalyl chloride with H3PO3 instead.
[Edited on 3-11-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
It is probably better to let this thread return to its OT focus (making Ac2O) rather than continuing this tangent about making POCl3, albeit for that
purpose.
See Inorganic Phosphorus Transformations in the Chemistry in General forum
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
after reading nicodems post on preparing benzoyl chloride from tcca and benzaldehyde I can not see why sodium acetate acetaldehyde and tcca in
methylene chloride at 40 C for three hours will not work.
the patent he posted was FR2633616 (A1)
e3500 console login: root
bash-2.05#
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Well, for starters I am skeptical of patents unless there is supporting peer reviewed literature available and accessible.
Secondly, assuming that aldehydes and TCCA give acyl chlorides, which is news to me, then I would recommend preforming the acetyl chloride and
reacting it with anhydrous NaOAc in a second step.
Finally, this would best be done in a pressure vessel since acetaldehyde is low boiling and it is going to be hard to reflux it without losses through
condenser for three hours over a 40 C pot, even if you use a Dewar condenser.
In the end if it does work just add it to the LONG list of well documented methods of making AcCl and Ac2O which have been elaborated in this thread
and prior threads on same subject.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Ephoton
after reading nicodems post on preparing benzoyl chloride from tcca and benzaldehyde I can not see why sodium acetate acetaldehyde and tcca in
methylene chloride at 40 C for three hours will not work.
the patent he posted was FR2633616 (A1) |
Well, if you would actually read the patent instead of generalising the reaction, you would notice that only nonenolisable aldehydes can be oxidized
to acyl chlorides this way.
Sauron, the radical chlorination and bromination of nonenolisable aldehydes with N-haloamides and other such reagents to give acyl chlorides and
bromides is well documented in the literature and industrial patents. The aldehydic hydrogen is easily abstractable and thus aldehydes often react in
reactions based on radical mechanism (aldehydes even react with oxygen; for example the oxidation of benzaldehyde to benzoic acid on air, etc.).
Just a few examples:
With Cl2: WO2003072534, US20030130537, EP1176133, EP849253
With SO2Cl2: Tetrahedron, 25 (1969), 4363-4369.
With t-BuOCl: J. Am. Chem. Soc., 73 (1951) 702-704.
With NBS: Tetrahedron Letters (1979) 3809-3810.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Thanks, Nicodem,for the clarification.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
thanx my french is not the best.
what got me is the table they have and the examples.
under test 1 they state acetaldehyde with a 68% conversion
to acid halide.
due to my french being so bad I misunderstood.
what do they mean by this test.
[Edited on 14-11-2008 by Ephoton]
e3500 console login: root
bash-2.05#
|
|
|
quarterfinal
Harmless

Posts: 12
Registered: 14-10-2008
Location: tx
Member Is Offline
Mood: No Mood
|
|
i have been trying out the vinyl acetate method mentioneed in earlier threads and have succeeded in getting acetic anhydride . but the yield and
purity are pretty bad. the yield is about 10% and the purity is about 60% or max 70%. there is a lot of acetic acid mixed with the product and its
pretty hard to seperate with even careful fractional distillation.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Ephoton
thanx my french is not the best.
what got me is the table they have and the examples.
under test 1 they state acetaldehyde with a 68% conversion
to acid halide.
due to my french being so bad I misunderstood.
what do they mean by this test. |
I apologize. The French patent indeed states a 40-68% yield for acetaldehyde to acetyl chloride. It's been a few years since I checked it and seems
like I mixed it up with some other paper on RCHO -> RCOCl where they claimed the reaction is only efficient for nonenolizable aldehydes or else
side products resulting from alpha chlorination prevail. I will check the papers I have on this when I'll have more time. Meanwhile I checked on
SciFinder and there is only one oxidation of MeCHO to AcCl listed, namely this same patent, which is suspicious to say the least. Searching for
enolizable aldehydes to the corresponding acyl chlorides only gives a 3 other examples of which two are on less easily enolisable
alpha-chloroaldehydes (JP06056739 and Zhurnal Obshchei Khimii, 28 (1958) 3004-3008), and one uses the decomposition of dibenzoyl peroxide in
CCl4 to promote only the radical process and chlorinate isobutyraldehyde (Journal of the American Chemical Society, 69 (1947) 2916-2917).
Perhaps the dichloroisocyanuric acid (pKa 3.75 in water) which forms during the reaction is not acidic enough to catalyse the enolysation of
acetaldehyde, particularly in the highly nonpolar CCl4 where its solubility and acidity is lower. Most examples of RCHO->RCOCl reaction use Cl2
where the side product is HCl and thus enolisation and consequent alpha-chlorination is inevitable.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
So the bottom line is that the sole patent is dubious and the yield sucks.
Not a very promising route.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
only one way to find out for sure save the searching ill test the thing.
that is if time permits.
e3500 console login: root
bash-2.05#
|
|
|
| Pages:
1
..
18
19
20
21
22
..
45 |