organometallic
Hazard to Self
 
Posts: 53
Registered: 22-7-2007
Location: United Kingdom
Member Is Offline
Mood: No Mood
|
|
n,n-dimethyltryptamine by Eschweiler-Clarke methylation of tryptamine
On an anonymous imageboard I sometimes peruse, there is a user who claims to have synthesized DMT via an Eschweiler-Clarke methylation of tryptamine,
while somehow avoiding Pictet-Spengler ring closure. I paraphrase:
To the formic acid and tryptophan solution, add 37% formaldehyde in water, dropwise. Continue reflux for 8 hours. Extract once with naptha to remove
traces of turpentine from a previous stage and discard this extraction. Basify to ph 10.5, do second naptha extraction. Cool this naptha extract
overnight in a freezer, filter out and dry DMT.
"As for why this method favors methylation over ring closure, the main thing is it occurs in the presence of a hydride source. What decides if there's
ring closure or methylation is whether the electrons of the indole double bond or the hydride get to the imine first, respectively, so you basically
want to make hydrides as available as possible to snatch the imine away from the indole's electrons." And this is done with a large excess of formic
acid, which acts as a hydride source apparently, although I personally do not understand how. Would someone briefly explain this?
"The ratio of Formic Acid:Tryptamine:Formaldehyde was 7:1:3. by mass"
and then...
"http://www.bluelight.ru/vb/showthread.php?t=375993
according to that link, tetrahydrobetacarboline (which is a synonym for Tryptoline) may be a potent dopamine neurotoxin, in the same way MPP+ is, aka
one dose and poof, parkinsons. While this route seems to produce mainly DMT, some tryptoline is almost CERTAINLY also being made and getting carried
over to the final product, so a decent purification scheme needs to figured.
The melting point of the crystals recovered was ~46 degC, while that of tryptoline and tryptophan are both >200degC.
Yes, I realise that this post is horribly sketchy and detritus-style.
My question is simply, what are your thoughts on this? It seems somewhat revolutionary..
In vials of ivory and coloured glass
Unstoppered, lurked her strange synthetic perfumes,
Unguent, powdered, or liquid - troubled, confused
And drowned the sense in odours.
|
|
|
tapira1
Hazard to Others
  
Posts: 168
Registered: 9-10-2006
Location: Here!!!
Member Is Offline
Mood: 
|
|
dimethyl trip
If you are aware of the mechanism of the EC and the PS reactions you will realize that you cannot avoid the PS process. No way...
|
|
|
organometallic
Hazard to Self
 
Posts: 53
Registered: 22-7-2007
Location: United Kingdom
Member Is Offline
Mood: No Mood
|
|
Anyone else please confirm/deny this?
"you cannot avoid the PS process"
In vials of ivory and coloured glass
Unstoppered, lurked her strange synthetic perfumes,
Unguent, powdered, or liquid - troubled, confused
And drowned the sense in odours.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by organometallic
Anyone else please confirm/deny this?
"you cannot avoid the PS process" |
Compare the reaction conditions with the ones stated in the Wiki article on the P-S rxn:
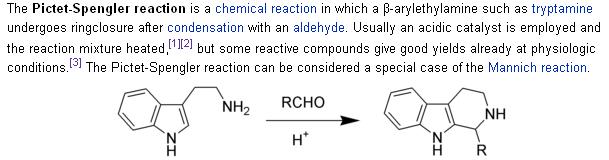
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by organometallic
On an anonymous imageboard I sometimes peruse, there is a user who claims to have synthesized DMT via an Eschweiler-Clarke methylation of tryptamine,
while somehow avoiding Pictet-Spengler ring closure. |
You read too much fiction.
Try reading scientific papers instead.
|
|
|
Barium
Hazard to Self
 
Posts: 85
Registered: 24-8-2008
Member Is Offline
Mood: No Mood
|
|
What Nicodem said...
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Unlike Benzaldehydes, Acetophenones usually don't react with nitromethane to form nitroalkenes. However, it was discovered recently, that when
ethylenediamine is used as the condensation catalyst, this reaction does occur. The diamine reacts simultaneously with both the Ketone and
Nitromethane, and brings the reactive intermediates into close proximity to one another. Then Viola! The Nitro-olefin is formed.
What does this have to do with DMT?
Read on.
Via the mannich reaction.....Acetophenone reacts with formaldehyde and Dimethylamine to form 3-Dimethylamino-Propiophen-1-one.
Use O-nitroacetophenone (Org. Syn.) as your starting material
and react the resultant O-Nitro, 3-Dimethylamino-Propiophen-1-one....with Nitromethane via ethylene diamine, and you instantly have a direct precursor
to DMT.
A simple reduction will reduce the nitro-styrene to an aldoxime,
while simultaneously reducing the O-nitro group to an amine.
Without further ado, the aldehyde and amine react to form the indole ring. And, bingo. DMT.
Will it work? Dunno. Just an odd dream I had. Maybe Barium has some input.
Edit 1: Organic Syntheses, Coll. Vol. 3, p.305 (1955); Vol. 23, p.30 (1943).
β-DIMETHYLAMINOPROPIOPHENONE HYDROCHLORIDE
[Propiophenone, β-dimethylamino-]
From acetophenone, easy and in high yield.
Edit 2: Organic Syntheses, Coll. Vol. 4, p.708 (1963); Vol. 30, p.70 (1950).
o-NITROACETOPHENONE
[Acetophenone, 2'-nitro-]
[Edit by Ramiel - quadruple posting for the new record. I relent because you posted references <i>etc.</i> please be aware of the edit
feature in future.]
[Edited on 7-9-2008 by Ramiel]
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
Haha quadruple posts, and they were all supposed to be one. Edit button works by the way 
Thanks for your input and all though.
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ah, I'll try that. When I open another window, to cut something out....to paste into my post, I return to find my stuff has been erased. New here.
I suppose I'll get the hang of it.
|
|
|
tapira1
Hazard to Others
  
Posts: 168
Registered: 9-10-2006
Location: Here!!!
Member Is Offline
Mood: 
|
|
acetophenone + diamine + formaldehyde
The reaction of acetophenone and derivatives with a N,N-diamine (not N,N'-diamine) and formaldehyde has long been known. It has even been described in
detail in one of the earliest volumes of Organic Reactions (your β-DIMETHYLAMINOPROPIOPHENONE HYDROCHLORIDE reaction) and is the subject of many
patents, such as that of the not so widely used today pridinol (patent is in German). This will work (the less water in the medium the better);
however, the nitromethane (nitroaldol/Henry) part looks as having poor prospect, even with ethylenediamine mediation.
Ethylenediamine was used as the acetate by Barton as promoter of the Henry reaction with benzaldehydes.
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Originally posted by zedAcetophenones usually don't react with nitromethane to form nitroalkenes. However, it was discovered recently, that
when ethylenediamine is used as the condensation catalyst, this reaction does occur. |
Will you please attach this paper or at least give a reference for it?
The reduction to indole with Fe/GAA is well known and is demonstarted in some of the papers from this thread
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
The reference I have seen is to a similar reaction of nitromethane with a ketone. I'll see if I can dig it up.
Boy, stuff gets buried fast.
A year or two back, folks were fairly skeptical about such reactions. They were considered hard to pull off. I remember the ethylene diamine
reference...because a researcher reported it as working, and his premise as to why it worked, seemed to make sense.
I've scanned the web a little, and at the moment, I'm finding other reagents that are claimed to effect the condensation between ketones and
nitroalkanes. Still, I think I'd bank on ethylene diamine first. I'll work on it.
|
|
|
McLovin382
Harmless

Posts: 19
Registered: 26-9-2008
Member Is Offline
Mood: No Mood
|
|
K gots some questions on this that could use answering...
Most importantly --- is Tryptoline really a dopamine neurotoxin? (Could someone point towards some references?) There is little information on the
compound on the 'net and the wiki says nothing about it. Think I read earlier actually that it's found naturally in some foods as well but I'm not
sure of the authenticity of that.
Also, would Formic acid be a suitable reducing agent if other alkylating agents (such as methyl iodide for instance) were used instead of
formaldehyde? The pictet-spengler reaction seems to require an aldehyde to take place.
Also, how much chance is there for the pictet-spengler reaction to happen? Are there ways to prevent such cyclizations? If any DMT would be formed it
could be filtered off in liquid form since tryptoline's boiling point is that much higher.
Thanks in advance
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Yup! Nitromethane will definitely react with Propiophenone, in a Henry-type condensation. Whether or not, it will produce a usable intermediate with
O-Nitro-Beta-Dimethylaminopropiophenone....Well, that remains to be seen.
Tamura, Sato, and Oda, report that under the influence of NH2CH2CH2N(CH3)2, Nitromethane may be readily condensed with ketones to produce
Nitro-olefins.
J. Org. Chem. 1986, 51, 4368-4375 Facile Synthesis of Allylic Nitro Compounds by N,N-Dimethylethylenediamine-Catalyzed Condensation of Aliphatic
and Alicyclic Ketones with Primary Nitroalkanes.
The Alpha Nitro-Alkene, formed from the condensation of Propiophenone and Nitromathane, may however isomerize to a product that might be inappropriate
for indolization. Hard to say.
Under the conditions the authors employed, regular Propiophenone condensed with Nitromethane to produce a Nitro-Olefin that isomerized to
C6H6-CH(CNO2)CH=CH2.
What O-Nitro-Dimethylaminopropiophenone, might do under these reaction conditions is difficult to project.
If the condensation can be achieved, and if the Nitro-Olefin can be captured in a favorable isomer.....Then Bingo! Upon reduction it should yield
Dimethyltryptamine.
O-Nitro-C6H5-C(=C-NO2)CH2CH2N(CH3)2 +H2-------->DMT
Don't know if it's been tried.
[Edited on 3-8-2010 by zed]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
In my experience, the Mannich reaction on ortho-nitroacetophenone did not work under the conventional conditions under which plain acetophenone
reacted normally (amine hydrochloride, 35% formaldehyde, ethanol reflux - essentially the conditions as described at Org. Synth.). I did not use
dimethylamine though (this was years ago, but if I still remember correctly I did try with diethylamine as well). The starting ketone was recovered
almost quantitatively (I think there has been a few % of conversion to the desired aminoketone, but I do not remember much about it any more). I can't
remember where, but I recently read a case where o-nitroacetophenone was said to fail some other reaction where enolization is also required. On the
other hand, there are at least two papers where this ketone is used successfully used in a Mannich reaction, so perhaps it is just a matter of adding
more HCl, using paraformaldehyde or whatever other detail... I have not checked what exactly, because I latter gave up on that synthesis but here are
two references if someone else wants to check: the o-nitroacetophenone Mannich reaction with piperidine is described in Archiv der Pharmazie,
316 (1983) 707-712, while with dimethylamine/paraformaldehyde in Angewandte Chemie IE, 46 (2007) 4489-4491.
The altered reactivity could also prevent any successful Knoevenagel condensation with nitromethane via the method in that JOC paper. Though, it
should be noted that o-nitromandelonitriles also cyclisize to the corresponding indoles upon hydrogenation (one example: Journal of Labelled
Compounds and Radiopharmaceuticals, 24 (1987) 637-643). It should be easier to prepare the cyanohydrin from
3-dialkylamino-ortho-nitropropiophenones than the nitromethane condensation product.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Thank you Nicoderm,
I'll check those Mannich reactions out, on my next visit to the library.
I have considered the route via the cyanohydrin, but for the moment, I am against it.
Messy stuff, Cyanide. Makes me nervous.
Of course, at the moment, the entire enterprise is a "Thought Experiment". A hypothetical romp. Currently dependent upon how folks fared during
those Mannich Experiments you quoted.
Piperidine in Archiv der Pharmazie, 316 (1983) 707-712,
Dimethylamine/paraformaldehyde in Angewandte Chemie IE, 46 (2007) 4489-4491.
|
|
|
Vogelzang
Banned
Posts: 662
Registered: 26-4-2008
Member Is Offline
Mood: No Mood
|
|
Did you see this?
https://www.hyperlab.info/inv/index.php?s=&act=ST&f=...
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. Transmethylation of tryptamine via a betaine. Sounds like a good idea. But, does it work? That someone smoked the product of such an
experiment, and they felt it got them "high", is not entirely convincing evidence. I wouldn't dismiss the claim out of hand, because it is possible.
But, I'd be a lot more impressed, if a product was isolated, and melting point information was recorded.
Even if they only succeeded in producing N-Methyl-tryptamine in good yield, that would really be quite an achievement.
|
|
|
starman
Hazard to Others
  
Posts: 318
Registered: 5-7-2008
Location: Western Australia
Member Is Offline
Mood: No Mood
|
|
Given the abundance of this substance in nature and the relative ease of isolation is it really worth the effort?Conversion to the 4hydroxy compound
via fenton/udenfriend would be more interesting.
Chemistry- The journey from the end of physics to the beginning of life.(starman)
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Yes, I believe it's worth the effort. A simple, high yielding, fairly non-hazardous route to N,N-Dialkyl tryptamines would be welcomed by many. The
guys in Big Pharmacy are surely interested.
Folks have been pounding away at synthesizing Tryptamine derivatives for a long time, and from a lot of different angles. A lot of progress has been
made in various areas, but a straightforward slam dunk hasn't arrived yet.
|
|
|
pacifyer
Harmless

Posts: 2
Registered: 20-7-2010
Member Is Offline
Mood: No Mood
|
|
Not sure if already explored, but maybe you can try a tryptophan double methylation, followed by decarboxylation.
|
|
|
Xalexalex
Harmless

Posts: 1
Registered: 22-12-2010
Member Is Offline
Mood: No Mood
|
|
I'm resuming this topic to ask your opinion on this variation of the Eschweiler-Clarke reaction, applied to tryptophan dimethylation: http://www.sciencemadness.org/talk/files.php?pid=86049&a...
Has anyone tried it yet? Do you think that the slight change in mechanism could actually avoid the Pictet-Spengler cyclization?
BTW, this is my first post here, hello all =)
|
|
|
Bolt
Hazard to Others
  
Posts: 188
Registered: 26-3-2007
Member Is Offline
Mood: No Mood
|
|
If you are looking for an MPTP --> MPP+ type neurotoxin, tryptoline is not, unless it is methylated in vivo. However, the product of the pictet
spengler condensation of N-methyltryptamine with formaldehyde could produce a toxic metabolite if the ring is then enzymatically dehydrogenated (by
MAO) to the pyridinium.
|
|
|
bfesser
|
Thread Split
24-7-2013 at 16:32 |