| Pages:
1
2
3 |
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
| Quote: | Originally posted by Vitus_Verdegast
2. At room temperature dehydration of 3-phenyl-1-propanol yields allylbenzene as the main product. For example, treatment of allylbenzene with SOCl2
yields chiefly allylbenzene and a small amount of propenylbenzene.
ref: Compt. Rend. 186 (1928) p. 1301, 1626, 1848
|
i couldn't find any experimental part in that reference.
|
|
|
swip2
Harmless

Posts: 6
Registered: 29-1-2008
Member Is Offline
Mood: Fuck all ya"ll.....
|
|
refs
Could those refs be posted
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
| Quote: | Originally posted by stoichiometric_steve
| Quote: | Originally posted by Vitus_Verdegast
2. At room temperature dehydration of 3-phenyl-1-propanol yields allylbenzene as the main product. For example, treatment of allylbenzene with SOCl2
yields chiefly allylbenzene and a small amount of propenylbenzene.
ref: Compt. Rend. 186 (1928) p. 1301, 1626, 1848
|
i couldn't find any experimental part in that reference. |
Attachment: Franch journal allylbenzene synthesis info.pdf (403kB)
This file has been downloaded 2021 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
| Quote: | Originally posted by swip2
Could those refs be posted
Mood: Fuck all ya"ll.... |
Right backatcha. Perhaps you could download it yourself from Gallica like stoichiometric_steve did.
Related some to some posts in this thread...there is another dehydration of hydrocinnamyl alcohol in the literature that I'm aware of.
But it is as an ester and the product was allylbenzene.
Hydrocinnamyl acetate, b.p. 133° (18 mm.) (61 g., 0.34 mole) was passed over glass wool heated at 460-485°. The pyrolysis product was washed with
aqueous sodium carbonate solution, dried and distilled at atmospheric pressure. The yield of allylbenzene, b.p 150-160°, was 31 g. (76%).
-JACS 78, 584 (1956)
|
|
|
swip2
Harmless

Posts: 6
Registered: 29-1-2008
Member Is Offline
Mood: Fuck all ya"ll.....
|
|
Well the link is all good and everything but Its In French ffs  .. ..
Is it possible for anyone to translate it, Or provide a link to an english version...
Dose stoichiometric_steve have it in english.
And could S.C. Wack please provide a refrence to the hydrocinnamyl alcohol comment please.
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
i can read french pretty well but it has NO experimental section.
|
|
|
no1uno
Harmless

Posts: 30
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
There is at least one reference to researchers having a-brominated cinnamaldehyde to the 2-bromo, which was reduced with yeast to the wanted
a-bromophenylpropan-3-ol. The researchers in question claim seriously high enantio-ratios, in using this process for making
phenylalaninol/phenylalaninal (I think Solo will know the reference I refer to*). Yeast reduction seems to be a useful route to enantiospecificity,
which in the case of such chiral compounds might make it worth a look.
Once you have n-methylphenylalaninol then a number of options open up, the scientific interest would be in the economical production of
anti-retroviral agents (which is what the paper deals with). It would be nice if someone could come up with a synthesis for pirating such agents,
given that the pharm. companies continue to charge incredible prices (well beyond the means of most sufferers).
* IIRC the part-title makes reference to 'Yeast reduction of cinnamaldehyde derivatives...'?
|
|
|
WizardX
Hazard to Self
 
Posts: 61
Registered: 11-8-2005
Location: wizardx.4shared.com
Member Is Offline
Mood: wizardx.suddenlaunch3.com
|
|
http://wizardx.t35.com/achive/cinnamic.html
The site in question was violating our TOS and was removed.
I'll upload it again soon.
[Edited on 20-8-2008 by WizardX]
Albert Einstein - \"Great ideas often receive violent opposition from mediocre minds.\"
|
|
|
zgoat65
Harmless

Posts: 11
Registered: 27-1-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | | Quote: |
I would say the reduction to cynamyl alcohol (or its aquisition given that it's pretty cheap), substitution with conc. HCl to yield cynamyl chloride,
reduction with Zn/AcOH to yield 1-phenylpropene from which the route proceeds by well known reactions. |
Would this be a valid route to (ref attached) cynnamyl chloride? This seems like the Holy Grail of OTC routes
Attachment: THE_REACTION_BETWEEN_ALCOHOLS_AND_AQUEOUS_SOLUTIONS_OF_HYDROCHLORIC_AND_HYDROBROMIC_ACIDS..pdf (671kB)
This file has been downloaded 1526 times
[Edited on 6-3-2012 by zgoat65]
[Edited on 6-3-2012 by zgoat65] |
zgoat65
|
|
|
zgoat65
Harmless

Posts: 11
Registered: 27-1-2012
Member Is Offline
Mood: No Mood
|
|
"There is a reference to the bromination of cinnamyl alcohol in Organic Synthesis (http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv5...).
The authors cite a 1918 German paper where it is claimed that cinnamyl bromide can been prepared from cinnamyl alcohol by the action of hydrogen
bromide in cold acetic acid. I'm don't know why these conditions wouldn't also brominate the alkene double bond. I don't have access to the paper so
I'm afraid I can't help more. You might be able to brominate the alcohol and the halide in one step. If you could selectively dehalogenate the
primary, you should be able to from the 2-Bromo-1-phenylpropane. You can then convert the halide to the iodide with the Finkelstein reaction in
acetone. (http://en.wikipedia.org/wiki/Finkelstein_reaction). Finally you should be able to aminate the iodide with methylamine in dry IPA as ++++ has shown
with iodosafrole."w
Taken from a thread elsewhere
[Edited on 7-3-2012 by zgoat65]
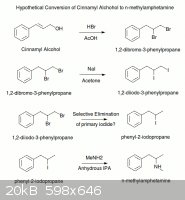
[Edited on 7-3-2012 by zgoat65]
zgoat65
|
|
|
cal
Hazard to Self
 
Posts: 88
Registered: 7-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sandmeyer  | Filemon, those links as provided do not prove your point. However, yield of propenylbenzene by the reduction of cinnamic aldehyde is 70% using
Huang-Minlon variant of Wolff-Kishner:
http://rapidshare.de/files/28334409/huang.pdf.html
I meen you can't go from that aldehyde for the sake of going from there, even if it involves hydrazine reduction, hinz even suggests Grignard. If this
starting material is to be used then I like Nicodems idea of making alcohol, then chloride, then OTC reduction. But to get to propenylbenzene you
could go from propiophenone (cheap, IMO probably the best single precursor if you're interested in simple stimulants [including phenmetrazine, which
at least Swedish speedfreaks prefer over methamphetamine according to Wikipedia, and one such person did report it better than amph on bluelight]
and have no regard for the law), reduce and dehydrate with tosic or sulfuric. Hell, there are other smoother ways of doing this, but amphetamine
dosen't interest me, so you find out..
That link does not exist anymore so if you have a copy, then post it and do not reference it.
[Edited on 6-8-2006 by Sandmeyer] |
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
"The authors cite a 1918 German paper where it is claimed that cinnamyl bromide can been prepared from cinnamyl alcohol by the action of hydrogen
bromide in cold acetic acid. I'm don't know why these conditions wouldn't also brominate the alkene double bond. I don't have access to the paper so
I'm afraid I can't help more. You might be able to brominate the alcohol and the halide in one step. If you could selectively dehalogenate the
primary, you should be able to from the 2-Bromo-1-phenylpropane. You can then convert the halide to the iodide with the Finkelstein reaction in
acetone. (http://en.wikipedia.org/wiki/Finkelstein_reaction). Finally you should be able to aminate the iodide with methylamine in dry IPA as ++++ has shown
with iodosafrole."w"
........in your last sentence...."Finally you should be able to aminate the iodide with methylamine in dry IPA as ++++ has shown with
iodosafrole."w"......do you have a reference for that statement?.......solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
zgoat65
Harmless

Posts: 11
Registered: 27-1-2012
Member Is Offline
Mood: No Mood
|
|
OR...........how bout this ref? Forget halogenation/dehalogenation, and go from alcohol to olefin using amalgamated zinc?
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6...
zgoat65
|
|
|
cal
Hazard to Self
 
Posts: 88
Registered: 7-2-2012
Member Is Offline
Mood: No Mood
|
|
License
Quote: Originally posted by Filemon  | | Quote: | Originally posted by Vitus_Verdegast
Be aware that in most countries it is a felony to produce and/or possess amphetamine without a license.
[Edited on 4-8-2006 by Vitus_Verdegast] |
Of course, they allow me microsynthesis but it should be immediately destroyed. |
Along with most all chemicals we want to buy now. That does not stop us from making what we need.
|
|
|
cal
Hazard to Self
 
Posts: 88
Registered: 7-2-2012
Member Is Offline
Mood: No Mood
|
|
Link
Quote: Originally posted by cal  | Quote: Originally posted by Sandmeyer  | Filemon, those links as provided do not prove your point. However, yield of propenylbenzene by the reduction of cinnamic aldehyde is 70% using
Huang-Minlon variant of Wolff-Kishner:
http://rapidshare.de/files/28334409/huang.pdf.html
I meen you can't go from that aldehyde for the sake of going from there, even if it involves hydrazine reduction, hinz even suggests Grignard. If this
starting material is to be used then I like Nicodems idea of making alcohol, then chloride, then OTC reduction. But to get to propenylbenzene you
could go from propiophenone (cheap, IMO probably the best single precursor if you're interested in simple stimulants [including phenmetrazine, which
at least Swedish speedfreaks prefer over methamphetamine according to Wikipedia, and one such person did report it better than amph on bluelight]
and have no regard for the law), reduce and dehydrate with tosic or sulfuric. Hell, there are other smoother ways of doing this, but amphetamine
dosen't interest me, so you find out..
That link does not exist anymore so if you have a copy, then post it and do not reference it.
[Edited on 6-8-2006 by Sandmeyer] |
|
Do you have this document as the download link is no longer available.
|
|
|
ScienceSquirrel
|
Thread Moved
25-3-2012 at 14:06 |
ScienceSquirrel
|
Thread Moved
26-3-2012 at 05:52 |
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Could you repost without using rapidshare? It's really volatile. That link was dead within a few days and I missed it.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
It's in any arcHive, with Huang-Minlon_propenylbenzene in the file title. Not convenient to quote from.
|
|
|
cmos6667
Hazard to Self
 
Posts: 50
Registered: 10-4-2015
Member Is Offline
Mood: No Mood
|
|
Dihydrocinnamyl alcohol dehydration
I read up on this and apparently you need either KHSO4 or conc. H2SO4 and high heat.
However, the product doesn't boil out, even being way past water's BP.
As far as I know, you should remove water but if you go beyond 80C, you get loads of side-reaction product.
Is it possible that (PhCH2CH2CH2)2O is formed and that's why you don't dehydrate?
In that case: can I take say 3 equiv EtOH and 1 equiv PhCH2CH2CH2OH to form the ethyl ether of dihydrocinnamyl alcohol (along with Et2O), then use
KHSO4 at 80C to eliminate EtOH while removing it and leaving behind allylbenzene?
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Why don't you post what you have read, as this section generally requires references?
|
|
|
cmos6667
Hazard to Self
 
Posts: 50
Registered: 10-4-2015
Member Is Offline
Mood: No Mood
|
|
Apologies, here: http://chemistry.mdma.ch/hiveboard/chemistrydiscourse/000204...
Before you get any wrong idea: the alkene is at the end position, so wacker or performic or whatever won't work, you need a catalyst under inert
atmosphere to rearrange it to the middle 
|
|
|
Mesa
Hazard to Others
  
Posts: 264
Registered: 2-7-2013
Member Is Offline
Mood: No Mood
|
|
You should probably add in that the beckmann will also not work on this substrate...
|
|
|
cmos6667
Hazard to Self
 
Posts: 50
Registered: 10-4-2015
Member Is Offline
Mood: No Mood
|
|
No carbonyl, no beckmann 
|
|
|
cmos6667
Hazard to Self
 
Posts: 50
Registered: 10-4-2015
Member Is Offline
Mood: No Mood
|
|
seriously though, any help here please?
|
|
|
Nicodem
|
Threads Merged
11-5-2015 at 11:38 |
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
My experience with acrolein tells, that you will get some solid gummy tar as a product, unless you distill your dehydrated product as fast as it is
formed.
Allylbenzene has b.p. 156-157°C, so this procedure can be performed pretty easily, until you condense the product back to where it will polimerize.
Those compounds are usually processed in gas phase via heterogenous catalyst, thus ensuring a short contact time.
PS: the problem is called "acid catalyzed alkene polimerization".
[Edited on 11-5-2015 by byko3y]
|
|
|
cmos6667
Hazard to Self
 
Posts: 50
Registered: 10-4-2015
Member Is Offline
Mood: No Mood
|
|
fair, solid input, thank you 
|
|
|
| Pages:
1
2
3 |