Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
The ant & the potato chip
I've lived in the same house for over 20 years, so both my habits & those of area insects are both very familiar.
This week brought a major change: I decided I enjoyed having potato chips with my mid-day sandwich. And a few days thereafter brought another but
apparently unrelated major change: an infestation of little black ants.
The ants were apparently driven to the Tupperware container that held the chips as they could be seen intently circling the plastic interface between
the top & main container.
It didn't take much thought to put together a working hypothesis for these apparently unrelated occurrences: the ants were being attracted from afar
by volatile chemicals given off by the chips.
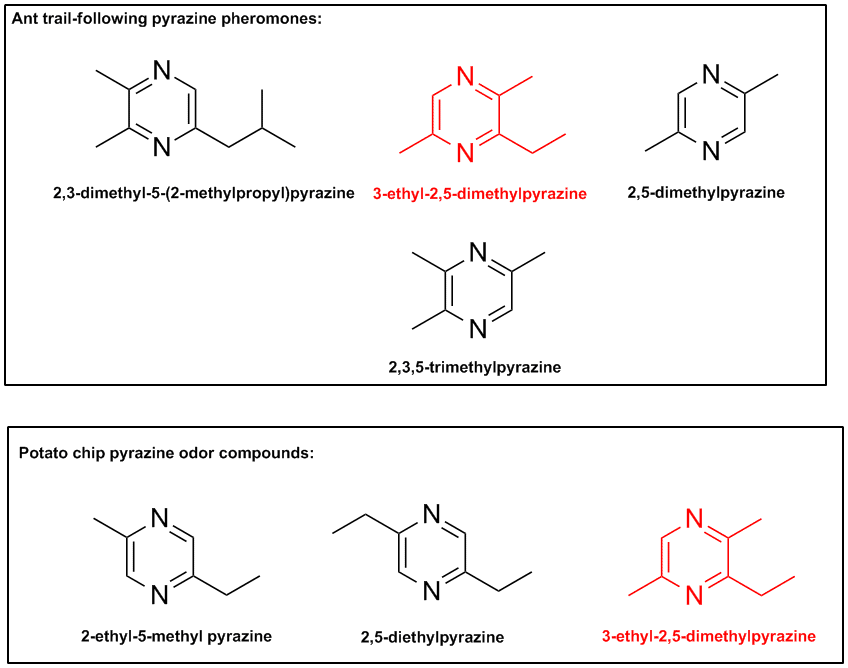
Since various alkylpyrazines are found both in ant trail-following pheromones & in potato chip volatiles, it didn't take much effort to establish
a correlation between the two. The chip's alkyl pyrazines, formed by the Maillard reaction when potatoes are fried, either mimiced or duplicated the
local ant species' pheromone that lead them to food sources such as my chips. See the amazing correspondence in the ChemDraw file.
Here is a reference for pyrazine potato chip volatiles: http://ift.confex.com/ift/2000/techprogram/paper_4447.htm
While the compound indicated in red might not be the exact culprit, ant pyrazine pheromones are known to be fairly general in their interspecific
attraction ability.
In the spirit of amateur experimentalism, I suggest that other subscribers try this same very simple experiment & report their observations here.
[Edited on 25-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
chloric1
International Hazard
    
Posts: 1146
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
The ant infestation phenominon is something of interest for sure. You could have a nearby colony that is so persistant that no pest control method
appears to work, then you change something and you never see another ant.
Case and pont-Me and my wife had a friend living with us in our condo and she had all of her non-refrigerated food and condiments in the ground level
cabinet. Starting this May, black ants infested our kitchen and we put ant bait, sprayed until we where seeing stars and they kept coming! Even a
single crumb or a dirty plate on the counter attracted the horde. As a last ditch effort I intentionally left out a slice of garlic toast overnight
to establish a "trail" of ants to find the source. To our frustration they originated from our friend/tenants stash and we insisted she THOROUGHLY
clean out the cabinet. The ants never returned even when we left something out overnight.
Fellow molecular manipulator
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
The room where the potato chip/ant problem occurred has a wall to the outdoors & I observed the ants getting in through a crack in the window
frame. A little pesticide & housecleaning eliminated the problem.
As a further test, the container with the chips is now in an inside room. No more ants overnight.
Given the size of these ants, these pyrazine compounds have to be active on the microgram level, so it's not surprising that the odor of chips carried
on the breeze through open windows was sufficient to attract them to the odor source.
[Edited on 26-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
-jeffB
Hazard to Others
  
Posts: 185
Registered: 6-12-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Ritter
Given the size of these ants, these pyrazine compounds have to be active on the microgram level, so it's not surprising that the odor of chips carried
on the breeze through open windows was sufficient to attract them to the odor source.
|
Picograms, actually:
http://www.eje.cz/scripts/viewabstract.php?abstract=1143
I'm picturing someone doing a normal lab-scale prep of one of these compounds for an unrelated reason, and returning the next day to find the entire
lab completely overrun with ants...
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
And interestingly the compound 3-ethyl-2,5-dimethylpyrazine seems to be a very common ant trail pheromone. It would not surprise me at all if this
chip volatile were indeed the culprit. It's also found in some cheeses & in coffee flavor.
A quick scan of my Aldrich catalog did not find a listing for it. I don't think it is a difficult synthesis. Some possibilities come to mind of how it
might be used as a 'prank' CW agent. But your point is valid: how to make macro scale quantities of a volatile insect attractant that is active on the
nanogram level without yourself becoming overrun?
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
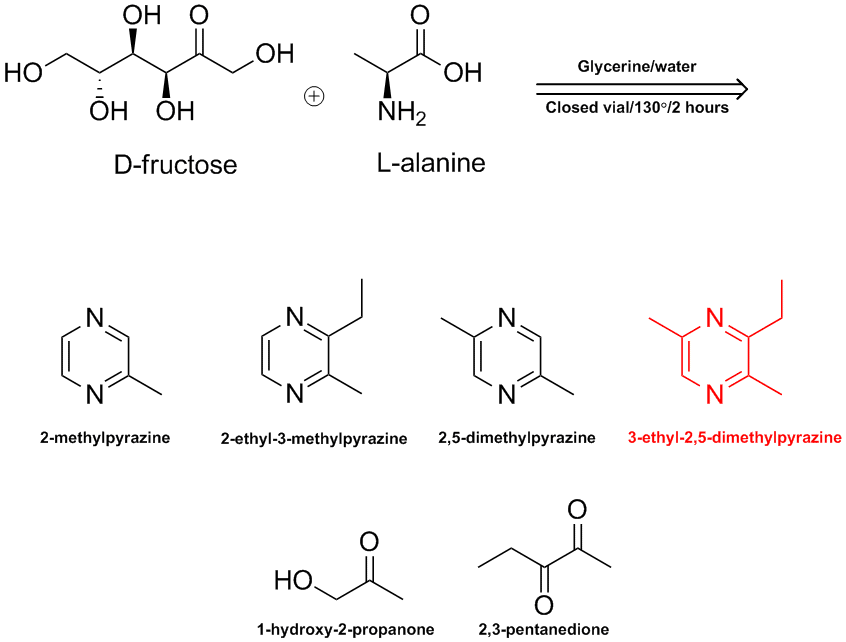
Pyrazines & other aroma chemicals arise when organic products such as potato slices are heated to high temperatures. A complex series of reactions
between the natural sugars & amino acids results in the formation of a number of aliphatic, aromatic & heterocyclic compounds in what is
called the Maillard Reaction.
In J. Agric. Food Chem., 54 (2), 574 -577, 2006 the Swiss aroma chemical company Firmenich study a model system as shown in the ChemDraw. No surprise
that 3-ethyl-2,5-dimethylpyrazine is one of the products produced.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
That is amazing!
Is anyone going to try to attract a bunch of ants by making this? I would like too, but i don't have any L-alanine, perhaps I may get some.
I am very interested in bug pheromones, because they have so much potential.
I Imagine a lot of bugs use them, perhaps some predatory bugs such as pray mantises, and lady bugs. attracting these sort of bugs could be very
beneficial for the gardener who, perhaps doesn't want to use poisons, but suffers from grasshopper and aphid problems.
Cockroaches could possibly be led into sticky traps with some of these synthetic bug pheromones.
I think it would be absolutely wonderful to make a chemical that brings in ants in by the millions 
And great observations and reasoning Ritter! 
Edit: Ladybugs obviously have a pheromone, because they will attract each other and hibernate in very large numbers. Perhaps a spray you put on plants
that attracts the bugs, is a million dollar idea! I also hear that the chemical can last a "long time" and cause them to hibernate in the same place
each year.
[Edited on 26-7-2008 by kclo4]
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | | Quote: | Originally posted by kclo4
I would like too, but i don't have any L-alanine, perhaps I may get some. |
I believe it is OTC in health food stores. DL-alanine would work just as well, AFAIK.
| Quote: |
I am very interested in bug pheromones, because they have so much potential. |
Yes, they do.
| Quote: |
I Imagine a lot of bugs use them, perhaps some predatory bugs such as pray mantises, and lady bugs. attracting these sort of bugs could be very
beneficial for the gardener who, perhaps doesn't want to use poisons, but suffers from grasshopper and aphid problems.
Cockroaches could possibly be led into sticky traps with some of these synthetic bug pheromones.
I think it would be absolutely wonderful to make a chemical that brings in ants in by the millions 
And great observations and reasoning Ritter! |
Thanks. Chemistry, like the other physical sciences, is all about observation & figuring things out.
| Quote: | | Edit: Ladybugs obviously have a pheromone, because they will attract each other and hibernate in very large numbers. Perhaps a spray you put on plants
that attracts the bugs, is a million dollar idea! I also hear that the chemical can last a "long time" and cause them to hibernate in the same place
each year. |
There is a very extensive literature on pheromones going back to at least the early 1970s. It's a great area for the amateur experimentalist to work
in.
|
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
Hmm, I will have to search for the types of pheromones ladybugs release.
From what I have read, some wasps, and rodents also use pyrazine derivatives.
So, if one did this synthesis or variations of it, they would probably run into more then just ants.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
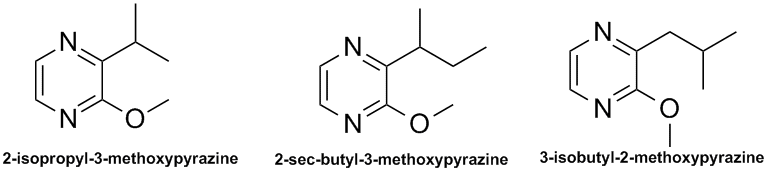
I found some references to pyrazine pheromones produced by ladybugs.
| Quote: | Headspace gas chromatography-mass spectrometry: a fast approach to the identification and determination of 2-alkyl-3- methoxypyrazine pheromones in
ladybugs.
Cudjoe E, Wiederkehr TB, Brindle ID
Analyst 2005; 130:152-5.
Abstract
Static headspace sampling technique coupled with gas chromatography and mass spectrometry was used to investigate the presence of volatile
2-alkyl-3-methoxypyrazines in three different species of ladybugs of the Coccinellidae family. The species investigated were Coccinella
septempunctata, Harmonia axyridis and Hippodemia convergens. 2-isopropyl-3-methoxypyrazine (IPMP) was identified in all three species
with detectable levels of 2-sec-butyl-3-methoxypyrazine (SBMP) and 3-isobutyl-2-methoxypyrazines (IBMP) in only Hippodemia convergens and
Harmonia axyridis species. Relative amounts of 2-alkyl-3-methoxypyrazines based on body mass showed that Hippodemia convergens had
the highest levels of all three methoxypyrazines and Coccinella septempunctata the least.
|
These involve slightly different chemistry than the ant/chip pyrazines:
[Edited on 26-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
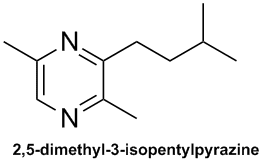
Sphecid wasps also produce a pyrazine attraction pheromone:
| Quote: | Pyrazines as marking volatiles in philanthine and nyssonine wasps (Hymenoptera: Sphecidae)
Journal Journal of Chemical Ecology
Publisher Springer Netherlands
ISSN 0098-0331 (Print) 1573-1561 (Online)
Issue Volume 6, Number 4 / July, 1980
DOI 10.1007/BF00990406
Pages 827-835
Subject Collection Biomedical and Life Sciences
SpringerLink Date Monday, January 10, 2005
PDF (1.1 MB)
Pyrazines as marking volatiles in philanthine and nyssonine wasps (Hymenoptera: Sphecidae)
A. -K. Borg-Karlson1, 2 and J. Tengö1, 2
(1) Department of Organic Chemistry, Royal Institute of Technology, S-100 44 Stockholm, Sweden
(2) Ecological Station of Uppsala University, Ölands Skogsby, S-386 00 Färjestaden, Sweden
Received: 28 September 1979 Revised: 14 December 1979
Abstract Males of several sphecid wasps apply volatile secretion on specific sites, perches. The odor is supposed to act in premating behavior as an
attracting pheromone. There are strong indications that the scenting material is produced by the mandibular glands. One philanthine species,
Philanthus triangulum, and three nyssonine ones, Argogorytes fargei, A. mystaceus, and Nysson spinosus, the latter
cleptoparasite on Argogorytes wasps, have been analyzed by gas chromatography-mass spectrometry. The major compound in the mandibular gland
secretion of the four species was tentatively identified as 2,5-dimethyl-3-isopentylpyrazine. A few other pyrazines not yet identified were also
found. Preliminary tests withP. triangulum show that alkylpyrazines influence male behavior.
Key words Pyrazines - 2,5-dimethyl-3-isopentylpyrazine - marking volatiles - mandibular gland - philanthine wasp - nyssonine wasp - premating
behavior - Hymenoptera - Sphecidae
|
This pheromone is very similar to the ant/chip compound discussed earlier in this thread.
[Edited on 26-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Ritter
I found some references to pyrazine pheromones produced by ladybugs.
| Quote: | Headspace gas chromatography-mass spectrometry: a fast approach to the identification and determination of 2-alkyl-3- methoxypyrazine pheromones in
ladybugs.
Cudjoe E, Wiederkehr TB, Brindle ID
Analyst 2005; 130:152-5.
Abstract
Static headspace sampling technique coupled with gas chromatography and mass spectrometry was used to investigate the presence of volatile
2-alkyl-3-methoxypyrazines in three different species of ladybugs of the Coccinellidae family. The species investigated were Coccinella
septempunctata, Harmonia axyridis and Hippodemia convergens. 2-isopropyl-3-methoxypyrazine (IPMP) was identified in all three species
with detectable levels of 2-sec-butyl-3-methoxypyrazine (SBMP) and 3-isobutyl-2-methoxypyrazines (IBMP) in only Hippodemia convergens and
Harmonia axyridis species. Relative amounts of 2-alkyl-3-methoxypyrazines based on body mass showed that Hippodemia convergens had
the highest levels of all three methoxypyrazines and Coccinella septempunctata the least.
|
These involve slightly different chemistry than the ant/chip pyrazines:
[Edited on 26-7-2008 by Ritter] |
Thanks, great find!
Well, now how would these be made?
I'm guessing different amino acids or different sugars? I don't know the mechanism of the reaction, heck, I hardly know the reaction of how they form,
so I wouldn't be able to predict what could form, but I bet you could make other pyrazine derivatives with other sugars.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Thanks, great find!
Well, now how would these be made?
I'm guessing different amino acids or different sugars? I don't know the mechanism of the reaction, heck, I hardly know the reaction of how they form,
so I wouldn't be able to predict what could form, but I bet you could make other pyrazine derivatives with other sugars. |
I don't believe the 2-alkoxy-3-alkylpyrazines can be made via the Maillard reaction. I suspect that they are either made by methylating
3-alkyl-2-pyrazinols or methoxylating 3-alkyl-2-halopyrazines. A quick search didn't come up with any clever ways to prepare these.
[Edited on 27-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
chemkid
Hazard to Others
  
Posts: 269
Registered: 5-4-2007
Location: Suburban Hell
Member Is Offline
Mood: polarized
|
|
Though producing the pure compound would interesting (if not fascinating) its a bit out of most of our reach. Perhaps we can instead cultivate methods
of increasing of concentration of said scents in food like potato chips, starting with a few simple experiments and testing them with ants to see
which is most attractive.
Unfortunately i am out of my lab for the summer  . .
Chemkid
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by chemkid
Though producing the pure compound would interesting (if not fascinating) its a bit out of most of our reach. Perhaps we can instead cultivate methods
of increasing of concentration of said scents in food like potato chips, starting with a few simple experiments and testing them with ants to see
which is most attractive.
Unfortunately i am out of my lab for the summer  . .
Chemkid |
It would be nice if we could but a) the 2-methoxypyrazines do not result from the Maillard rxn AIAIK and b) the isopentyl pyrazine would require an
apprpriately substituted amono acid for it to be formed in that rxn & that would be a major project in its own right.
Here are references on the synthesis of 2,5-dimethyl-3-isopentylpyrazine:
| Quote: | Sato, N., and Matsuura, T. 1996. Studies on pyrazines. 31. Alkylation of chloropyrazine N-oxides by Nickel-catalyzed cross-coupling reaction with
dialkylzincs. J. Heterocycl. Chem. 33:1047-10490.
Sato, N., and Matsuura, T. 1996. Studies on pyrazines. Part 32. Synthesis of trisubstituted and tetrasubstituted pyrazines as ant pheromones. J. Chem.
Soc. Perkin Trans. 1:2345-2350.
|
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|