Zelot
Harmless

Posts: 45
Registered: 27-1-2008
Member Is Offline
Mood: hopeful
|
|
Non-nitrate explosives
I've used up all of my sulfuric/nitric acids at the moment, so I was wondering about non-nitrate explosives. Has anyone heard of chlorine-based
high-explosives? They sound like the easiest to produce in a pinch (minus the chlorates) from pool supplies. Any suggestions?
So... what did you do over the weekend?
|
|
|
Zinc
Hazard to Others
  
Posts: 472
Registered: 10-5-2006
Member Is Offline
Mood: No Mood
|
|
http://www.sciencemadness.org/talk/viewthread.php?tid=2945
|
|
|
AngelEyes
Hazard to Others
  
Posts: 187
Registered: 24-1-2003
Location: South of England
Member Is Offline
Mood: Better than it used to be.
|
|
Azides (-N<sub>3</sub> and Acetylides (-C<sub>2</sub> and Acetylides (-C<sub>2</sub> also spring to mind. also spring to mind.
Plus perchlorates, permanganates...etc.
[Edited on 11-6-2008 by AngelEyes]
\'Silk and satin, leather and lace...black panties with an Angel\'s face\'
|
|
|
ScienceGeek
Hazard to Others
  
Posts: 151
Registered: 22-1-2008
Location: Norway
Member Is Offline
|
|
AngelEyes:
Could one say that perchlorates and permanganates are true explosives? I.e., they're not just powerful oxidising agents, but explosives just by
themselves...+
|
|
|
Microtek
National Hazard
   
Posts: 872
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Organic perchlorates such as ethylperchlorate are certainly very powerful (and very sensitive) explosives, when the organic radical is suitably small.
|
|
|
Zinc
Hazard to Others
  
Posts: 472
Registered: 10-5-2006
Member Is Offline
Mood: No Mood
|
|
Are there any organic permanganates?
|
|
|
bereal511
Hazard to Others
  
Posts: 162
Registered: 9-8-2005
Location: Madison, WI
Member Is Offline
Mood: No Mood
|
|
I believe tetrabuytlammonium permanganate is a relatively common one. I doubt it's explosive to any extent though.
I stand corrected.
http://orgprepdaily.wordpress.com/2008/03/16/tetrabutylammon...
[Edited on 11-6-2008 by bereal511]
As an adolescent I aspired to lasting fame, I craved factual certainty, and I thirsted for a meaningful vision of human life -- so I became a
scientist. This is like becoming an archbishop so you can meet girls.
-- Matt Cartmill
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Benzyltriethylammonium permanganate (C6H5CH2(C2H5)3MnNO4) explodes around 80 to 90 deg.C.or from heavy blows, and can ignite spontaneously. The
trimethyl compound also explodes around the same temperature. There are a few others like these. Organic permanganates are even less stable than the
perchlorates, if the lower alkyl permanganates even exist then they are extremely unstable.
[Edited on 11-6-2008 by Schockwave]
|
|
|
Zelot
Harmless

Posts: 45
Registered: 27-1-2008
Member Is Offline
Mood: hopeful
|
|
In the thread zinc directed me to, I found a theoretical explosive that would be fun to try-out: chlorinated isopropylamine. Sounds easy to make, with
OTC precursors. You first make isopropylamine by bubbling ammonia gas through rubbing alcohol in a copper pipe. Then you chlorinate it as with the
other amines and you're done. Any thoughts? Anyone willing to try this?
So... what did you do over the weekend?
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Others I can think of, not listed above (i.e. excluding nitrates, nitro-compounds, chlorates, perchlorates, permanganates): bromates, perbromates,
tetrazolates, fulminates (isocyanates) of heavy metals, organic peroxides, nitrogen trichloride/tribromide/triiodide.
[Edited on 11-7-08 by JohnWW]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Fulminates are different from isocyanates:
Fulminic acid is formonitrile oxide, hydrocyanic oxide:
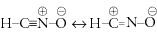
Fulminic acid and soluble fulminates have similar toxicity to cyanides.
Isocyanic acid is:
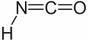
this stuff is also not very stable. In the gaseous state it smells pungent and causes lacrimation, when condensed it irritates the skin and gives a
large amount of pain under blister formation.
Nitrides (of some non-metals and heavy metals), organic ozonides, and polyacetylides would be some others. Not to mention the large amount of oxidizer
and fuel mixtures.
|
|
|
Zelot
Harmless

Posts: 45
Registered: 27-1-2008
Member Is Offline
Mood: hopeful
|
|
Speaking of oxidizer/fuel mixes, is chlorate/sugar able to detonate?
So... what did you do over the weekend?
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
I'm not sure if it's possible, it could be that the mixture only has deflagrating properties or it might be that there is a critical mass for a
mixture of KClO3 and sugar needed for them to detonate if they do. Mixtures with petroleum compounds like Explosif P, S and Minélites are known
detonable where KClO3 was even used in WWI in mixtures as an explosive filler.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Has anyone tried dissolving N2O in a fuel? It's said to be "very soluble" in ethanol and ether, though I have no figures. That should be like a
detonable fuel-oxygen mixture. Methanol might also work and could be more powerful. N2O is not too reactive, or toxic but a strong oxidizer, the same
wooden splint test for pure oxygen is said also to work with N2O.
|
|
|
pdb
Hazard to Self
 
Posts: 90
Registered: 8-4-2004
Member Is Offline
Mood: No Mood
|
|
You could add to your list the cheddites, based on mixtures of chlorates with diverse stuff, and so named after Chedde, which is a small city in
France were chlorates were industrially prepared by electrolysis (located in the Alps, electricity would come from hydraulic sources).
The name became a trademark of an explosives company which doesn't exist anymore. However, the trademark has been retained by another company,
specialized in percussion caps for rifles cartridges. I like their logo, kept from the former company:
(center)(img src="http://www.sciencemadness.org/scipics/pdb/cheddite.jpg")(/center)
|
|
|
Leander
Harmless

Posts: 28
Registered: 23-2-2008
Member Is Offline
Mood: No Mood
|
|
Cheddites are cheap, simple and sensitive, but not really powerfull. KClO3 with a plain fuel has a VoD of 3000- 3500 m/s. KClO3 might be a a strong
oxidiser but there are much more factors necessary to make a good explosive. The reaction surface between the fuel and its oxidiser is really small
just like ANFO, and the mixture contains over 60% inerts, because of the potassium ion. KClO3/sucrose can be detonated, but with a pretty large
critical diameter, and it will probably suffice a large booster.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Peroxochromates are another.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
nitrogen halides, NI3 NCl3. very easy to make but sensitive as hell  oh and
nitrogen trichloride is light sensitive! just to add to the fun oh and
nitrogen trichloride is light sensitive! just to add to the fun 
|
|
|
Blasty
Hazard to Others
  
Posts: 107
Registered: 25-7-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Leander
Cheddites are cheap, simple and sensitive, but not really powerfull. KClO3 with a plain fuel has a VoD of 3000- 3500 m/s. KClO3 might be a a strong
oxidiser but there are much more factors necessary to make a good explosive. The reaction surface between the fuel and its oxidiser is really small
just like ANFO, and the mixture contains over 60% inerts, because of the potassium ion. KClO3/sucrose can be detonated, but with a pretty large
critical diameter, and it will probably suffice a large booster. |
By adding certain chemicals to the chlorate/sugar mixtures you can make them detonate even by using a comparatively small flash powder charge. Berge's
blasting powder, for example:
(scanned original, page 330)
http://books.google.com/books?id=8BdDAAAAIAAJ&printsec=f...
(transcribed version)
http://chestofbooks.com/reference/Henley-s-20th-Century-Form...
The smaller amount of potassium chromate speeds up the reaction. The wax is apparently added in order to make the mixture less sensitive to shock and
friction.
I have experimented a bit with Berge's blasting powder without the wax (I will do some experiments with the wax one later on.) The mixture burns very
fast, in a flash. If you confine it in a tube and set it off with a fuse it explodes in a black powder-like manner (but with more force, of course.)
But if you put a blasting cap (no "booster", just a plain fulminate or HMTD cap) or even a confined flash powder charge inside it, the shock will make
it detonate (I have detonated samples in Tylenol plastic bottles even by using flash powder firecrackers. You must be careful that the fuse of the
firecracker does not come in contact with the charge of Berge's blasting powder and ignite it prematurely. You want the flash powder explosion itself
to set off the charge, not the sparks or flame from the fuse.)
[Edited on 26-7-2008 by Blasty]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by Schockwave
Has anyone tried dissolving N2O in a fuel? It's said to be "very soluble" in ethanol and ether, though I have no figures. That should be like a
detonable fuel-oxygen mixture. Methanol might also work and could be more powerful. N2O is not too reactive, or toxic but a strong oxidizer, the same
wooden splint test for pure oxygen is said also to work with N2O. |
Nevermind this. The MSDS says supercritical N2O in mixture with ethanol or methanol can be detonable. N2O isn't readily soluble in methanol, and
ethanol most likely also not.
|
|
|
sakshaug007
Hazard to Self
 
Posts: 94
Registered: 11-3-2009
Location: USA-Pacific Northwest
Member Is Offline
Mood: No Mood
|
|
There's always the copper acetylide and silver acetylide compounds that are extremely explosive and easy to synthesize. Just bubble acetylene gas
through a solution of silver chloride and the product precipitates out.
AgCl + C2H2 = Ag2C2 + HCl
Ag2C2 = Ag(s) + C(s) + BOOM!
|
|
|
a_bab
Hazard to Others
  
Posts: 458
Registered: 15-9-2002
Member Is Offline
Mood: Angry !!!!!111111...2?!
|
|
Silver chloride doesn't form solutions, as it's notoriously insoluble (just like BaSO4).
And even if it were soluble, acetylene alone wouldn't be able to take the Cl ion out of the chloride salt. An ammoniacal complex is needed for this
(in order to render the salt soluble - works for copper). In practice, silver nitrate is used.
This has been discussed to death here, though. And reviving dead threads is not advisable.
Other than that, welcome 
|
|
|
sakshaug007
Hazard to Self
 
Posts: 94
Registered: 11-3-2009
Location: USA-Pacific Northwest
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by a_bab
Silver chloride doesn't form solutions, as it's notoriously insoluble (just like BaSO4).
And even if it were soluble, acetylene alone wouldn't be able to take the Cl ion out of the chloride salt. An ammoniacal complex is needed for this
(in order to render the salt soluble - works for copper). In practice, silver nitrate is used.
This has been discussed to death here, though. And reviving dead threads is not advisable.
Other than that, welcome  |
My bad, I mixed up the analogous copper chloride synthesis of copper acetylide with this silver acetylide synthesis. Thanks for the correction, and
uhh... I'll put the thread to rest, sorry.
|
|
|