| Pages:
1
2 |
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Cu/SiO2 a cheap versatil hydrogenation catalyst?
While seaching information on the selective 1,4-hydrogenation of unsaturated ketones ("Raspberry ketone" thread), I stumbled on the use of 8% Cu/SiO2
catalyst as a selective, easily made hydrogenation catalyst. Further enquiry showed that by changing reaction conditions, preparation conditions, etc,
this catalyst could be usefull for reduction of ketone to alcohols, deoxygenation of aromatic ketone to methylen compounds (so long dirty Clemmesen
and Wolf-Kishner!), and of course selective 1,4- hydrogenation of unsaturated ketones, without producing the corresponding alcohol or other side
products...
The catalyst is easily prepared, by adding silica to a solution of Cu(NH3)4 2+ (made by adding conc. ammonia to Cu(NO3)2.3H2O solution, and diluting
the slurry to hydrolyse the complex and deposit a finely dispersed precipitate of Cu(OH)2. Filtration, washing and drying prior to calcination at
350°C and reduction by H2 at 270°C under atm pressure afford a finely dispersed Cu/SiO2, non pyrophoric or pyrogenic catalyst.
This catalyst is commonly used in the industry, and can be recycled several times. I'm sure it can be usefull for other applications than those cited
previously, possibly dehalogeantion, reduction of nitro compounds, etc. By modifying the loading and the preparation, it's selectivity can be changed.
This could turn out to be a versatil, easily made catalyst for the home-chemist, opening doors to reactions that otherwise require expensive and/or
pyrophoric catalyst such as 10% Pd/C, raney nickel, etc.
I decided on trying out this catalyst on the 1,4-hydrogenation of an unsaturated ketone, to compare the selectivity and efficienty to Pd/C, and then
try other applications. Different grades of silica will be tried.
Preparation of 8% Cu/SiO2
Preparation of the unreduced catalyst
Cu(NO3)2.3H2O solution was prepared by adding fine 3.5g (55.12 mmol) Cu pellets (Panreac) (washed with acetone and dH2O prior to addition) to 13mL
(143.27 mmol) 53% w/w HNO3 (technical) (excess Cu). there was vigorous evolution of NO2, and significant heating up as the solution quickly turned
blue. the erlenmeyer was then heated to 50°C for 12H, and left to rest at RT for another 24H.
This resulted in a slightly viscous cyan blue solution, with a little amount of unreacted copper. The solution was decanted of the metal, and
completed with a little dH2O to 10mL (~12g/10mL : 1200g/L solution).
Concentrated solution (1200g/L):

Diluted solution (160g/L):

3.3mL of this solution were diluted to 25mL with dH2O (160g/L solution). 30% NH4OH (Panreac) was then added until the Cu(OH)2 precipitate that
initially formed redissolved. pH was >9, and a very dark blue solution was obtained.
30% NH4OH:

Cu(OH)2 precipitate:

Cu(NH3)4 2+ solution:

10g of chromatographical grade silica gel (60-200mesh, Acros) were then added in portions, with slow magnetic stirring. there was significant warming
up of the solution. The dark blue slurry was stirred for 30min at RT.
Silica gel:

Slurry:

The slurry was then transfered to a 1L beaker placed in a ice bath, and slowly diluted to 1L with strong magnetic stirring. The blue color cleared up
to a dark cyan blue colour. After 10min stirring, the blue solid was left to decant, leaving a very slightly blue supernatant. Half of the solution
was filtered off, and the remaining supernatant diluted to 1l with stirring. After decantation, the now colorless supernatant was filtered off,
leaving the solid in the beaker, and the first filtrate added, and diluted to 1L with stirring. The solid was then vacuum filtered, thoroughly washed
with 200mL, and dried by suction for 5min.
First dilution:

Decanted solid:

Filtered solid:


The solid was then transfered to a pyrex dish, and heated on the hotplate to ~100°C. The caked solid quickly dried back to a very fluid powder, as
dried silica gel, and was left at 100°C overnight. There were a few light white particules, which was considered to be talc from the latex gloves..
Wet solid:

Dried solid:

In the morning, the solid still was cyan blue. It was transfered to a 250mL beaker, heating increased to 150°C for 1H, the solid turning slightly
darker, then placed on a heating mantle and slowly heated to 350°C. The solid gradually turned dark green, and was kept at that temperature for 4H.
After 12H drying:

Calcination:



The catalyst will be reduced prior to the reaction, by atmospheric H2 at 270°C. The formed water will be removed either by vacuum distn, or by
refluxing toluene with a Dean Stark (the reaction will be performed in toluene at 90°C)
References: (Available in the Ref's forum)
Thank very much to Nicodem, who very kindly recovered most of the articles.
Cu/SiO2: an improved catalyst for the chemoselective hydrogenation of a,b-unsaturated ketones
Nicoletta Ravasio, Marisa Antenori, Michele Gargano and Piero Mastrorilli
Tetrahedron Letters 37(20), 3529-3532 (1996)
Selective hydrogenation of 4-(6-methoxy-2-naphtyl)-3-buten-2-one to Nabumetone®
Nicoletta Ravasio, Federica Zaccheria, Pietro Allegrini and Mauro Ercoli
Catalysis Today, Volume 121, Issues 1-2, 15 March 2007, Pages 2-5
Heterogeneous selective catalytic hydrogenation of aryl ketones to alcohols without additives
Federica Zaccherianext , Nicoletta Ravasiob, Rinaldo Psarob and Achille Fusi
Tetrahedron Letters 46(21); 3695-3697 (2005)
Cu/SiO2-catalyzed hydrogenation of cyclohexanones under very mild conditions
Ravasio, N.; Psaro, R.; Zaccheria, F.
Tetrahedron Letters 43(21); 3943–3945 (2002)
Heterogeneous Cu-catalysts for the reductive deoxygenation of aromatic ketones without additives
Federica Zaccheriaa, Nicoletta Ravasiob, Mauro Ercolic and Pietro Allegrini
Tetrahedron Letters 46(45), 7743-7745 (2005)
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Dr.3vil
Hazard to Self
 
Posts: 59
Registered: 27-7-2006
Location: Secret Underground Bunker
Member Is Offline
Mood: 4He + 8.7 MeV
|
|
Nice work! I am looking forward to seeing the writeup for the reduction of the catalyst by H2. So far this seems like a great way to open up
hydrogenation to the home chemist.
DR.3vil
- Paper chemistry works the first time every time -
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Thank you! I hope this catalyst can be used for lots of different reactions involving hydrogen, giving a very cheap and safe substitute to Raney
Nickel, Pd/C, copper chromite, etc
The final weight was 10.5g, so it seems not all the copper was absorbed, and that the catalyst hasn't got an 8% loading. Thsi could be du to thet fact
that I added a little too much ammonia, and the the volume of water required for complete hdrolysis was superior to what I added. In one of the
articles, they dilute the complex's solution to 3L!
Next time i will add the ammonia dropwise, or maybe use excess Cu salt to compensate.
The silica was initially dry (I can through it in the oven for 30min at 150°C to be sure of the weight), so I would assume at most 0.1g silica loss,
giving 0.6g of absorbed CuO (7.5 mmol) giving just under 0.5g Cu when reduced, so a 5% loading.
I will moniter H2 uptake during activation of the catalyst (after flushing the setup with argon, then H2 to compensate for the volume of the setup),
to compare results. I will use a little more catalyst during reductions to obtain the same substarte/cu ratio as the ones used in the articles.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Attempted reduction of dehydrozingerone by Cu/SiO2-catalyzed hydrogenation
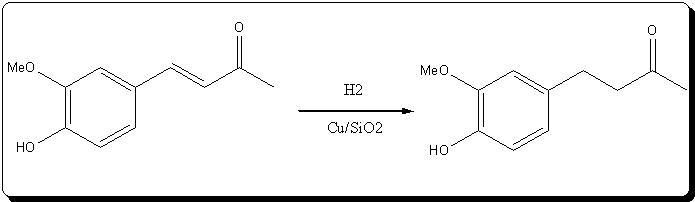
The catalyst prepared above was used in the atm hydrogenation of 4-(4'-hydroxy-3-methoxyphenyl)but-3-en-2-one ("dehydrozingerone") according to
Ravasio et al. (see ref above).
The catalyst was easily reduced by atm hydrogen at >250°C, and the substrate as a toluene/AcOEt solution under argon was introduced. reduction was
performed at 90°C with atm hydrogen for 24h.
Reduction of the catalyst
A 100mL 3-neck 14/19 flask was equipped with the following: a simple distn setup with no vacuum inlet (receiver directly attached to the condenser), a
claisen adaptator fitted with: a gas inlet connected to a hydrogen generator (pressure equalized addition funnel on 4-neck 250mL RBF), a gas admission
tube connected to an inverted 250mL cylinder immersed in a beaker of water; a septum.


0.90g (Ratio substrate/catalyst ~5% mol/mol) of the previously prepared catalyst were weighed and introduced in the flask, with a stir bar.


[IMG]http://img.photobucket.com/albums/v622/Amine
/Zingerone/CuSiO2_Hydrogenation/Cat_Red1.jpg[/IMG]
The whole system was purged with argon 3 times, then the H2SO4 was drippe donto the Zn slurry, and the system purged with H2 two times. The cylinder
was filled with H2, and the flask was heated with a mantle to 250-300°C, stirring and shaking the fluid catalyst, which gradaully darkened over
15min. H2 absorption started very quickly, and stopped after ~15min. Heating and stirring was continued for another 15min. A few small water droplets
had deposited in the top of the necks.



Under a stream of argon, the distn setup was removed, and the condenser fitted between the flask and the claisen. The setup was purged with H2 3
times.
Reduction of the unsaturated ketone
The unsaturated ketone was introduced in a schlenk, and 30mL distilled toluene were added. Not all the ketone dissolved, so ~5mL AcOEt were added to
acheive complete dissolution. The very light yellow solution was degassed 3 times, and transfered to the reduced catalyst by canula. The system was
briefly purged with H2, and the cylinder refilled.


The flask was heated to 90°C in an oil bath, and vigorously stirred under H2 atmospher for 24h, withdrawing samples with a a syringe evry 6h. After
24H, the mixture had taken a green colour.

Unfortuanly, TLC revealed that no reduction had taken, not the slightest little spot wher ehte saturated ketone should ahve been... No alcohol,
unsaturated alcohol, just a tiny un-eluted spot from condensation products I guess.....
I'm not sure about the reason for this complete inactivity of the catalyst. I can only suppose the kind of silica is truely determinating... Most of
the articles mention "fumed silica", I never thought it would have such different properties from chromatographic silica... IIRC, chromatographic
silica is prepared by acidification of sodium silicate, or hydrolsyis of Si(OCH3)4, while pyrogenic silica is prepared by combustion/hydrolysis of
SiCl4. Which properties one has that the other one hasn't is behond me, but I thinkit has something to do with the stabilisation of copper hydride
species.
I have written to Mrs. Ravasio asking her if she has encoutered similar problems with different grades of silica...
Until then, I will start looking elsewhere, NiB catalyst to be precise. These could be easily made from Ni salts and NaBH4,a nd are said to be very
active, non-pyrophoric selective catalyst. An improved caatlyst can even be prepared from used NiMH batteries! the variosu lanthanes, Cobalt, Mn
species present exhalte the reducting properties.. Until then, back to paperwork...
See:
Hydrogenation catalysts from used nickel metal hydride batteries
Mark R. St J. Foreman, Christian Ekberg and Arvid Ödegaard Jensen
Green Chem. 2008, 10, 825 - 826,
Abstract
A synthesis of a safe and effective hydrogenation catalyst has been developed with used nickel metal hydride electrical cells as the starting
material.
availble at the ref forum.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Barium
Hazard to Self
 
Posts: 85
Registered: 24-8-2008
Member Is Offline
Mood: No Mood
|
|
Nice work Klute!
Even if you didn't get the result you were hoping for you still got some valuable experience and that is priceless.
I haven't had any success with any of the "cheap n' easy" versions of the classic catalysts. The Raney type (RaNi, RaCo, RaFe, RaCu) and the precious
metal
catalysts are, so far, the ony ones which have behaved as supposed. Urushibara nickel, nickel boride, cobalt boride, you name it. I've tried them all
and all have failed. But I might just be a world class bum when it comes to prepare them. Who knows?
[Edited on 28-9-2008 by Barium]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Thank you Barium, I am very honored.
Would you care elaborating on your experience with metal borides? Did you prepare the collodial catalyst? or supported/polymer-protected? in water, or
EtOH?
I am going to purchase some NiSO4.7H2O for preparing nickel complexes anyway, so might just aswell give it a try. The PEG-protected and
Mg(OH)2-supported catalyst looked very promising, but so did the Cu/SiO2... i know that the Cu/SiO2 when correctly prepared works well, some
colleagues have used commercial 30% for dehydrogenations with great results. I guess it's the quality of silica that counts, and pyrogenic silica is
expensive and cancerigen, so it ruins the point of getting easily made, cheap and safe catalyst somewhat..
Here are some related refs:
Colloidal nickel boride on rare earth oxides for hydrogenation of olefins
Yu-Lin Jiang 1, Xue-Yun Wei, Shou-Ping Tang and Liu-Bin Yuan
Catalysis Letters; 34 (1995) 19-22
available at the ref froum
Abstract:
An extremely active and colloidal nickel boride was prepared by using rare earth oxide as a
support in the presence of polyethylene glycol (PEG) at room temperature which could act as
a catalyst in the hydrogenation of olefinic compounds, such as 4-(41-hydroxyphenyl)-3-buten-
2-one.
Preparation and catalytic activity of colloidal nickel boride dispersed in ethanol
Yukimichi Nakao and Shoei Fujishige
Chemistry Letters; (8)8, 995-996 (1979)
Pdf
Abstract:
Colloidal nickel borides in ethanol were prepared by the reaction of nickel(II) chloride with sodium borohydride in the presence of a polymer like
poly(vinylpyrrolidone). The colloid was effective for the hydrogenation of acrylamide at 30°C under an atmospheric hydrogen pressure.
A highly active supported catalyst for olefin hydrogenation from collodialNickel Boride
Yukimichi Nakao
Chemistry Letters; (7)11 , 997-998 (1982)
Pdf
Abstract:
Supported catalysts, which were prepared from colloidal nickel boride by immobilizing on inorganic substances such as Mg(OH)2, exhibited higher
activity for olefin hydrogenation than a sol-type catalyst of colloidal nickel boride protected by polyvinylpyrrolidone.
Looks very promising, especialyl if NiMH batteries can be used as starting material!
[Edited on 28-9-2008 by Klute]
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Before giving up it might be worth following the procedures outlined in literature more closely...especially catalyst loading, this seems to be
important for even common hydrogenation catalysts.
Although, I am a little suspicious of this catalyst based upon the fact that this research seems to be done by a small group of individuals; all the
papers you referenced contain at least 1 common researcher.
Nice writeup.
[Edited on 9-28-2008 by smuv]
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I'm slightly confused, so I just want to check something... You are hydrogenating your substrate yes? But you say
| Quote: |
some colleagues have used commercial 30% for dehydrogenations with great results
|
Is that right? Because I think it's either a typo ( incrediably likely compared to the other option I'm about to propose ) or you're using a DEhydrogenation catalyst for hydrogenation? ) or you're using a DEhydrogenation catalyst for hydrogenation?
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Cu/SiO2 is pretty commonly used in the industry, so I have no doubt it works.
It can be used as a hydrogenation AND dehydrogenation catalyst depending on the conditions (just like palladium for example): it can also be used as a
CTH catalyst (with IPA, dehydrogeantion of IPA to acetone, and hydrogeantion of an substrate, or hydrogenation of acetone and dehydrogenation of a
substarte for example).
I have contacted to the author, N. Ravasio, which replied to me very quickly. She confirmed that pyrogenic silica is not required to have an active
catalyst, but her guess is that the phenol interfers with the freshly reduced, reactive copper metal. I wil try using an alkyl ether, or using
straight benzalacetone (which they have succesfully reduced)
The catalyst loading used was that of the procedure in the article, the only difference was that soem AcOEt was added to acheive complete dissolution
of the substrate. Mrs Ravasio thinks AcOEt can slow the reduction. She has been helpfull, and I must say I am rather impressed as most of the authors
I have already contacted when failing their procedures simply never answered or didn't care about my results, blaming bad lab technic.
I will use the remaining prepared catalyst with other substrates, but wil also start working with NiB catalysts.
News to come
[Edited on 29-9-2008 by Klute]
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Barium
Hazard to Self
 
Posts: 85
Registered: 24-8-2008
Member Is Offline
Mood: No Mood
|
|
Klute,
I have prepared both P1 and P2 nickel boride in different ways. P1 was prepared in the usual way by adding 1M aqueous sodium borohydride with powerful
stirring to 0.5M aqueous nickel(II)chloride. I tried it immediately in both a stirred hydrogenation vessel at about 8 bar pressure and in a CTH
reaction at STP using potassium formate as the hydrogen donor.
The P2 nickel boride was prepared trying EtOH, diglyme and (if I remember correctly) N,N-dimethylacetamide as solvents. The P2 catalyst made in these
solvents was tried in the same reactions as the P1.
If I remember correctly the substrates in the hydrogenations was 1-(2,4-dimethoxyphenyl)-2-nitroethane and 1-(5-methoxy-1H-indol-3-yl)-2-nitroethane.
In all cases the result was crap, nada, null! As a result of this I never tried nickel boride with any other substrate.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Ah, but I don't recall any mention of nitro grousp being reduced by NiB... Did you read anything ont he subject, or did you just assume that it would
work seeing that NiB catalyst are often compared to Pd/C or Raney Nickel?
I'm going to try the Cu/SiO2 reduction on methyalted phenylbutenones, and am still waiting for my nickel chloride has Acros were out of stock lately.
I will keep you informed when I get things started. Didn't you trt reducing the nitroalkenes to nitroalkanes?
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Barium
Hazard to Self
 
Posts: 85
Registered: 24-8-2008
Member Is Offline
Mood: No Mood
|
|
D. E. Nichols, Synthesis of alpha-Alkyltryptamines, J. Org. Chem., 51, 4294-4295 (1986)
http://www.erowid.org/archive/rhodium/chemistry/it-290.html
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
| Quote: | Originally posted by Barium
In all cases the result was crap, nada, null! |
Same for me. I much prefer using NaBH4 and CTH in sequence.
|
|
|
Barium
Hazard to Self
 
Posts: 85
Registered: 24-8-2008
Member Is Offline
Mood: No Mood
|
|
Steve, which substrate have you tried nickel boride on?
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Ok, I've finally bought some NiSO4.6H2O as the chloride was taking too long to getin stock, I will start some hydrogenations next week. I will start
with phenolic phenylbutenones, and then maybe try out some alcenes, and might give a try with unsaturated nitro compounds, but from you guys
experiences, I'm not expecting much out of it. I will surely prepare the NiB supported on CaCO3, and eventually polymer-protected. This eems to have
quite an influence on the absorption rate.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
kmno4
International Hazard
    
Posts: 1502
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Cu/SiO2 sounds nice....
But I see your reduced catalyst and I do not see any metallic Cu in it. I saw reduction powdered CuO by H2 and black material turn into bright-red
one. I think your catalyst should behave similary
Zn/H2SO4 is not good solution, because of traces H2S in H2 stream. I would use Al/NaOH (currently I am building electrolyzer for H2 generation, pure
and wasteless way for hydrogen).
*********
reduced Cu on SiO2 should be deep violet color, not bright Cu as I though.
[Edited on 21-10-2008 by kmno4]
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
| Quote: | Originally posted by Barium
Steve, which substrate have you tried nickel boride on? |
Not allowed to say  I'd much rather use Zn/H+ any day... I'd much rather use Zn/H+ any day...
|
|
|
starman
Hazard to Others
  
Posts: 318
Registered: 5-7-2008
Location: Western Australia
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by stoichiometric_steve
| Quote: | Originally posted by Barium
Steve, which substrate have you tried nickel boride on? |
Not allowed to say  I'd much rather use Zn/H+ any day... I'd much rather use Zn/H+ any day... |
could you post some details over on the zinc thread?
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
starman,
If you were truly interested in the matter you would have made use of the search engine of the forum.
I have long ago laid out the conditions for a real one-pot reduction of nitroalkenes using NaBH4 and Zn/H+ in sequence (work based on Bandils posts at
the Hive).
|
|
|
FrankRizzo
Hazard to Others
  
Posts: 204
Registered: 9-2-2004
Member Is Offline
Mood: No Mood
|
|
Klute,
Fumed silica can be found commercially as product called Cab-O-Sil. Taxidermy supply houses usually carry it.
|
|
|
Barium
Hazard to Self
 
Posts: 85
Registered: 24-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by stoichiometric_steve
| Quote: | Originally posted by Barium
Steve, which substrate have you tried nickel boride on? |
Not allowed to say  I'd much rather use Zn/H+ any day... I'd much rather use Zn/H+ any day... |
Can you say if it was a phenyl-2-nitroethene, phenyl-2-nitroethane, phenyl-2-nitropropene or phenyl-2-nitropropane? I dont care about any substitution
patterns on the ring.
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
Ba: the latter.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Thank you for the comment KMnO4. I too was pretty surprised byt he color, but I just considered thatt he low catalyst loading gave that color. I am
pretty sur ethe copper has been reduced, as there was the theorical amount of H2 absorbed, no more, no less, and it was very rapid. I will try out new
variations, using non-phenolic substartes pretty soon.
Communications with the author revealed that fumed silica is not at all mandatory, and that excellent results where obtained with chromatographic
silica, although the catalyst has a stronger acidity which can cause some condensations etc with delicate substrates (cyclisation of b-ionone). but
lower catalyst loadings still give perfect results with most substrates.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
I'm curious. Did you use acidic, neutral, or basic silica gel? Also, do you think it would be possible that your technical grade nitric acid
contained metal ions that could have poisoned the catalyst?
[Edited on 12/3/08 by bfesser]
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I used neutral chromatographical silica gel, well it has a certain acidity. The nitric acid has never posed any problems before, and even if there
would be some metals, it would only be very small traces...
I have contacted the main author of the series of publication, and she recons the problems comes from the phenolic substrate: the very fine Cu metal
is very reactive, requiring absence of water, so changes are it's the phenol function poisoining the catalyst.. I was going to alkylate the substrate
and try the reduction again, aswell as trying out the reduction of benzylidenacetone, a reaction N. Ravasio et al have done, to compare the results
with the same substrate, and see if the preparation of the catalyst (type of silica, dilution, etc) has a major influence on the selectivity and
yields, but I cannot work on this anymore. Feel free to try it out! It would be very interesting to see how this goes
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
| Pages:
1
2 |
|