Intergalactic_Captain
Hazard to Others
  
Posts: 228
Registered: 4-9-2004
Location: somewhere where i don\'t know where i am
Member Is Offline
Mood: frabjous
|
|
Suggestions for oil-bath oil?
I'm in need of a cheap bath oil that won't smoke or decompose in the 100-200degC range, preferably something availible at walmart. I could go with a
legitimate laboratory oil, but I don't like waiting for shipments.
I've been using ethylene glycol (there's always antifreeze laying around for some reason), but it's really only good if you're outdoors, upwind, and
preferably wearing a respirator. Due to the weather, I've got to move everything back indoors for the next couple of weeks. I was thinking of a
synthetic motor oil - Anyone care to comment or offer a better suggestion?
If you see me running, try to keep up.
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
Canola oil (or just about any vegetable based oil) works fine for temperatures up to 225*C or so (although I have taken it up to 250*C without any
problems).
The only bad? thing about using this is that it makes stuff smell like you're frying food. Hence the bad? 
[Edited on 16-5-2008 by PainKilla]
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
| Quote: | Originally posted by Intergalactic_Captain
I'm in need of a cheap bath oil that won't smoke or decompose in the 100-200degC range, preferably something availible at walmart. I could go with a
legitimate laboratory oil, but I don't like waiting for shipments.
I've been using ethylene glycol (there's always antifreeze laying around for some reason), but it's really only good if you're outdoors, upwind, and
preferably wearing a respirator. Due to the weather, I've got to move everything back indoors for the next couple of weeks. I was thinking of a
synthetic motor oil - Anyone care to comment or offer a better suggestion? |
You should be able to find mineral oil as a laxative in many pharmacies. As it is meant to be taken internally, it is quite pure and can even be used
as transformer oil. I believe the decomp temperature for mineral oil is 500C.
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
Shadow is dead on.
Get the heavy mineral oil laxative... it'll go at least 225ºC before it even begins to think about smoking.
Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in
beer.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
For heating around 180°C, one can use cold saturated aqueous calcium chloride solution, mineral oil is good around 200°.
Don't forget salt baths can also be used (usually for higher temperatures though), especially those with a low melting eutectic mixture of salts, or
melting salts alone. Between 100 and 200°, a salt would be good that melts in that area but does not decompose and should the heating apparatus
break, a compound that is not incompatible with the substance being heated .
If you have the CRC handbook you can flip through it and quickly find the melting points of some common solids, and then search to see their hazards
and decomposition points.
|
|
|
Intergalactic_Captain
Hazard to Others
  
Posts: 228
Registered: 4-9-2004
Location: somewhere where i don\'t know where i am
Member Is Offline
Mood: frabjous
|
|
All right, sounds like I'm going with the laxative...What am I looking at for cost per quantity? I'm guessing it isn't sold by the liter, and I'd
like to grab at least 2.
If you see me running, try to keep up.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
It's VERY expensive for how much you get so recycle it. You might have to buy 2 or 3 bottles. It usually comes in a clear plastic bottle and will
simply say, "Mineral Oil" on it.
Like I said, reuse that shit! It's not cheap!
In my area it's about $7 for a pint (473mL). At least it is very pure (USP I believe).
[Edited on 5-16-2008 by MagicJigPipe]
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
Intergalactic_Captain
Hazard to Others
  
Posts: 228
Registered: 4-9-2004
Location: somewhere where i don\'t know where i am
Member Is Offline
Mood: frabjous
|
|
That's kind of what I figured, was hoping it would be a bit cheaper though. I guess I'll give canola oil a shot - worst comes to worst I'll just fry
up some chicken. No input on motor oil though? I'd imagine it would take the heat, but one thing I've learned over the years is just how
counterintuitively most things work out.
If you see me running, try to keep up.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Motor oil smells worse than ass. I don't know what typical smoke point is for various weights, but I would guess regular 10W-30 doesn't need to be
very high inside an engine regulated at 80-120C.
Tim
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
Yeah, I know for a fact synthetic blend 10w30 starts to smoke at around 150*C. At least.
And the smoke is horrible. I would stay away from it. Next time you've been driving for a long time open up your oilcap and look closely. You might
see a small amount of smoke coming out. I know I do.
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I'm using Dot 5 brake fluid -- expensive but doesn't breakdown or smoke at 100-200*C on a humid day, make a shield so your condenser doesn't drip
into it and get the condenser off to the side .. otherwisse lots of crackling going on.. no damage though .. annoying. I need to make a rubber pad
for my jackstand .. only slightly larger than the space occupied by the feet of my deep fat fryer.. the fryer keeps perfect temp stability!
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
How about using "Vaseline" petroleum jelly? I've never actually tried it but it is very pure, has a very high boiling point and melts around 100F.
Might be worthwhile if a high temperature is needed.. Just speculation though.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
I think it's just as much for what you get. Unless you get generic petrolatum then maybe... I wouldn't heat it with a flame, though. Also, for
temperatures up to 150*C, the optical lab I worked in used glycerol.
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
If you try the vetinary store for laxative (as opposed to human stuff) it will be much cheaper. It is called 'liquid paraffin' in my neck of the
woods. Used for pluged up calves etc. Can buy by the gallon. Not too sure if it is the same as mineral oil.
Dann2
|
|
|
bio2
Hazard to Others
  
Posts: 447
Registered: 15-1-2005
Member Is Offline
Mood: No Mood
|
|
Best heating oil for me is that hard, hydrogenated, solid frying "lard" mixture sold unrefrigerated by the kilo, wrapped in paper.
Some versions have a high percentage of cottonseed oil which when hydrogenated has perhaps the highest smoke point of any vegetable oil.
Spill proof when cool, won't smoke at 220 degrees, very cheap, usable for months and makes the lab smell like a french fryer restaurant.
The smell diminishes considerably after it has been used
at high temp for a while.
|
|
|
Intergalactic_Captain
Hazard to Others
  
Posts: 228
Registered: 4-9-2004
Location: somewhere where i don\'t know where i am
Member Is Offline
Mood: frabjous
|
|
Cheap wal-mart canola oil seems to boil somewhere around 140 C... I was trying to distill acetic acid from sulfuric and NaOAc and had to stop, the
takeoff rate was too slow when trying to keep the oil from boiling. I think I'll try a "crisco" type oil next, though the canola oil held up fine
under ~130 C.
If you see me running, try to keep up.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
Why not just use mineral oil? Why fuck with all of these flammable cooking oils? I don't understand that. Have you ever had a grease fire? It's
not fun...
Also, I've heard a mixture of some glycol monoethyl ethers and glycol ethers can be used as a high temperature bath oil. I have access to all of
these in bulk quantities (very cheap). Perhaps I shall try them.
EDIT
Also, I take back what I said about mineral oil being very expensive. It's only $2.50 a pint (473mL) for very pure USP grade mineral oil in my area.
It's even a little cheaper than that if you get the quart (946mL) size. If you buy a gallon or so (about $18) it should last you a VERY long time if
you reuse it. I mean, it's not like it's going to get very dirty as long as you use a clean bath and even if it does it's not that big of a deal.
Use that... I still don't know why nobody has mentioned glycerol. There must be SOME reason why the Lenscrafters optical lab uses it to heat the dye
solutions.
[Edited on 5-18-2008 by MagicJigPipe]
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
Yeah heavy mineral oil laxative works the best.
All the other organic oils such as vegetable and what not have a nasty tendency to polymerize.
Dino based oils smoke like hell.
Brake fluid can take some heat, but it absorbs moisture and gives off dangerous fumes when overheated.
Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in
beer.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
Yeah, I think that's what the brake fluid is ... The glycol alkyl ethers and probably some diethylene and triethylene glycol. In fact, I'm sure some
of them contain a majority of di-triethylene glycol.
Hmmmmm... I think triethylene glycol might be something to try by itself. It's boiling point is 285*C. It is hygroscopic but I don't think that
matters too much as you're going to be heating it anyway. Only thing I can think of is the actual vapors being toxic. Probably something to stay
away from now that I think about it. (that means stay away from brake fluid, too)
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Grocery store mineral oil will most definitely smoke and burn. It might be rather volatile at the temperature that you want. Depends on the mw. Same
with wax. Dudes of yesteryear used wax baths, but I'm not sure that Gulf Wax is so great at higher temperatures.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | | Originally posted by MagicJigPipe Use that... I still don't know why nobody has mentioned glycerol. There must be SOME reason why the
Lenscrafters optical lab uses it to heat the dye solutions. |
I've got a reference (Römpp) that mentions using glycerin for heating baths, they also say its good for heating around 170ºC, they also mention for
this purpose: concd. H2SO4 (for heating around 200º, though I wouldn't use this it's too dangerous, hot oil is one thing, hot acid, another);
diethylene glycol (for around 245ºC), paraffin (for 250ºC), triethylene glycol (276ºC), and the pentyl ester of silicic acid (360ºC). For
temperatures up to 400ºC, mixtures of biphenyl and diphenylether as well as ditolyl ether ("Diphyl"). For temperatures between around 200 to 700ºC, salt baths are employable.
It's also notable mentioning that there are salts which will increase the boiling point of water ("boiling point elevation"), so a salt can be added
which gets this effect, one of those is a CaCl2 solution mentioned earlier.
In each case it's important that the flash point of the compounds is not reached and to avoid the harmful gases where they are formed. Baths not using
glycols or glycerin are easier to keep anhydrous.
|
|
|
bio2
Hazard to Others
  
Posts: 447
Registered: 15-1-2005
Member Is Offline
Mood: No Mood
|
|
SC Wack is right!
Any of you guys using mineral oil at 200 degrees?
I tried it once years ago and never again. Fumes off badly and veg oil is much
safer at 200 degrees. Wax is just as bad.
Most of these more "unconventional" liquids mentioned have bad odors/fumes and that's
why most people never use them. A closed system is what most of these commercial
heat transfer fluids is intended for.
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
The mineral oil must be labeled as heavy which has a higher B.P. than normal "light" mineral oil.
Check out this chart:
http://www.sonneborn.com/literature/INT_white_oils_prop_char...
And yes you can buy it online for like $15 a gallon plus shipping:
http://www.chemistrystore.com/Kaydol35.htm
The product in the above link, Kaydol 35, has a flash point of 224ºC.
[Edited on 19-5-2008 by evil_lurker]
Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in
beer.
|
|
|
kalacrow
Harmless

Posts: 38
Registered: 23-5-2008
Member Is Offline
Mood: No Mood
|
|
http://www.ikea.com/us/en/catalog/products/00046786
Very inexpensive if you have an IKEA near you, but I do not know what the bp is for their product (mineral oils differ in their bp). for $4 you can
find out 
Oh one more thing.. I have a friend who is a potter, and they assure me that they can easily make me simple heating mantles (not with wire inside..
like the ones for placing atop heating elements) out of high temp ceramic for pennies, considering they are so "crude", by their standards. Find an
example of one online. and see if you can find a friendly potter to avoid the HUGE prices of commercial ceramic heating mantles.
[Edited on 27-5-2008 by kalacrow]
|
|
|
amrhamed2
Hazard to Self
 
Posts: 60
Registered: 14-12-2007
Member Is Offline
Mood: No Mood
|
|
a table
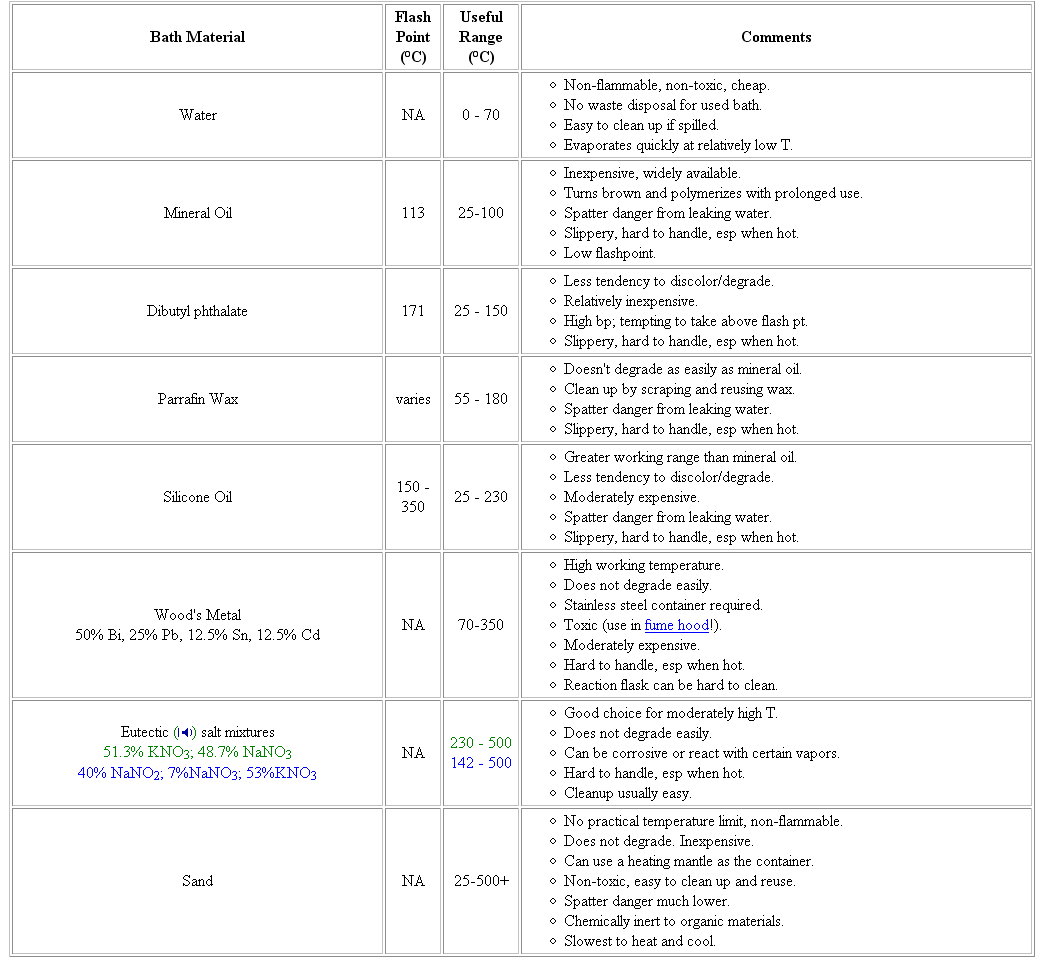
amr h mahmoud
|
|
|