| Pages:
1
2
3 |
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by franklyn
on urea ( urine ) precipitating NCl2_CO_NCl2 . I suppose the same holds true for guanidine yielding NCl_CNCl_NCL2, or any organic having primary
amines. |
No, urea, and probably guanidine form NCl3. Via what mechanism I'm not sure probably hydrolysis to CO2 & NHCl2 followed by further chlorination of
dichloramine yielding the yellow oil of NCl3.
[Edited on 10-6-2006 by Axt]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Could it be analogous to the haloform reaction?
Tim
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
http://www.wikimirror.com/Nitrogen_trichloride
Links and resources _
http://www.justnitrogen.com/nitrogentrichloride
U P D A T E
http://www.sciencemadness.org/talk/viewthread.php?tid=6717&a...
.
[Edited on 6-9-2007 by franklyn]
|
|
|
Zelot
Harmless

Posts: 45
Registered: 27-1-2008
Member Is Offline
Mood: hopeful
|
|
Sorry to bring up this thread, but I am interested in franklyn's post about urea forming a primary explosive with chlorine. Could you tell me more
about that?
So... what did you do over the weekend?
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
| Quote: | Originally posted by Axt
| Quote: | Originally posted by franklyn
on urea ( urine ) precipitating NCl2_CO_NCl2 . I suppose the same holds true for guanidine yielding NCl_CNCl_NCL2, or any organic having primary
amines. |
No, urea, and probably guanidine form NCl3. Via what mechanism I'm not sure probably hydrolysis to CO2 & NHCl2 followed by further chlorination of
dichloramine yielding the yellow oil of NCl3.
[Edited on 10-6-2006 by Axt] |
How did you manage to not see this?
It is also very unlikely as a compound, due to the strongly eletronegative carbonyl in the centre, destabilising the putative tetrachloro urea even
further.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Reply to Zelot
| Quote: | | Originally posted by Axt - No, urea, and probably guanidine form - - the yellow oil of NCl3. |
| Quote: | | Originally posted by chemoleo - It is also very unlikely as a compound, due to the strongly eletronegative carbonyl in the centre,
destabilising the putative tetrachloro urea even further. |
Apparently not since there is no mention of this in literature.
Dichlorourea has been known a long time
http://www.sciencemadness.org/talk/viewthread.php?action=att...
Not explosive itself, this decomposes slowly to form NCl3
which is very dangerous. http://www.lateralscience.co.uk/oil/index.html
.
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Originally posted by Axt - No, urea, and probably guanidine form NCl3. Via what mechanism I'm not sure probably hydrolysis to CO2 & NHCl2
followed by further chlorination of dichloramine yielding the yellow oil of NCl3. |
| Quote: | | Originally posted by chemoleo - It is also very unlikely as a compound, due to the strongly eletronegative carbonyl in the centre,
destabilising the putative tetrachloro urea even further. |
| Quote: | | Originally posted by franklyn - Apparently not since there is no mention of this in literature. |
From the article you just gave,
"... since, in the presence of HCl formed at the same time, hydrolysis so readily occurs and so much nitrogen chloride is produced..."
Apparently not? I'd say apparently it does, everything you gave supported it.
Scifinder when given the structure of tetrachlorourea provides no hits, though from memory I did find a pentachloroguanidine.
Interesting targets would be chlorinated products of glycine and sulphamic acid which would enable the inclusion of catalytic metal ions, it would at
least be interesting to see if this has any effect on explodability. Though chlorination and precipitation with lead/silver for example would be
difficult. Maybe a potassium salt depending on solubility. Both the free acids are known and freely soluble, HO-S(=O)2-NCl2 is probably susceptable to
hydrolysis as well.
[Edited on 20-3-2008 by Axt]
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Axt... though from memory I did find a pentachloroguanidine.
|
Here it is,
Chlorination of guanidine and cyanamide. Davydov, A. V.; Kretov, A. E. USSR. Zhurnal Vsesoyuznogo Khimicheskogo Obshchestva im. D. I.
Mendeleeva (1981), 26(4), 478-9. CODEN: ZVKOA6 ISSN: 0373-0247. Journal written in Russian. CAN 95:168503 AN 1981:568503 CAPLUS
Abstract
Guanidine nitrate was chlorinated in H2O-CHCl3 contg. finely divided marble and NaCl at -10° to give 37% ClN:C(NCl2)2. Chlorinating H2NCN with HOCl
in H2O-CCl4 contg. NaCl at -10° yielded 65% ClC(:NCl)NCl2 (I). Treating I with dry HCl in CCl4 yielded 85% HN:CXNH2.HCl (II; X = Cl), which reacted
with refluxing MeOH to give II (X = OMe).
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
They've been there done that
Pentafluoroguanidine is known and quite a super explosive in terms of its energy
product ≈ 3300 Kcal/ Kg , I am uncertain as to how it's detonation properties
compare to more prosaic oxo group materials.
http://www.sciencemadness.org/talk/viewthread.php?tid=6717&a...
Fluoroamino groups in a boron heterocyclic compound was examined many years
ago either by Los Alamos or Livermore, the exact reference I discarded because
of the disappointingly poor performance exhibited in the actual tests , although
characteristically for this type of functional group it's energy is paradoxically quite high.
B - Tri ( difluoroamino ) - N -Tri - fluoroborazole
∆Hf (Heat of formation) = -184 Kcal/ mol
Density ( ρ ) ≈ 1.7 gm/cm³
Detonation pressure . P = 212 Kilobar
Velocity of detonation . VOD = 6400 Meters/sec
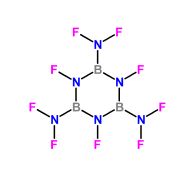
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by franklyn
I am uncertain as to how it's detonation properties
compare to more prosaic oxo group materials.
|
The attachment gives comparison to CF4, CO2, HF, H2O, Al2O3 and AlF3 detonation products. The conclusion was that only HF showed promise over H2O
where "blast over brisance" is desired, but then isnt that what aluminium is for. CF4 and AlF3 "do not seem to be worth the trouble".
EDIT: Was too big to attach.
[Edited on 14-4-2008 by Axt]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Here's another from PATR:

This is from Beilstein (Vol. 1, p. 1154):

Diethylenedichlorodiamine C4H8Cl2N2 = ClN.C4H8.NCl. Prep.: from diethylenediamine and NaClO. The precipitate is suctioned off immediately. – Strong
smelling columns (from alcohol). Melting point: 71 deg.C. Explodes at 80 deg.C. Sparingly soluble in water, barely soluble in ether, easily in
alcohol. Upon standing in water, diethylenediamine hydrochloride forms.
[Edited on 20-4-2008 by Schockwave]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Here's some more from Beil:

Chloropiperidine C5H10ClN = C5H10.NCl. Formation. By mixing clear, aqueous solutions of piperidine and chloride of lime. - Pungent smelling oil. After
several hours (and through heat, explosively) it changes into piperidine chloride. It boils with some decomposition at 52 deg. at 25 mm. Very volatile
with water vapours. By prolonged boiling with water, piperidine results. Warming it with conc. HCl releases chlorine.

Naphthoquinone chloroimide. C20H10NClO3 (?). Formation. By treating a-amido-a-napthalene with chloride of lime solution. - Bright-brown needles (from
dilute acetic acid). Meltpnt.: 85 deg. Explodes at 130 deg. Barely soluble in water, easily in alcohol, ether and acetic acid.

... Diacetyl-o-bischloroaminobenzene C10H10O2N2Cl2 = C6H4(NCl.CO.CH3)2. Prep.: by shaking diacetyl-o-phenylenediamine (see above) with a potassium
bicarbonate containing solution of hypochlorous acid. quadrilateral prisms. Melts at 94 deg. with a light explosion. It isomerizes in glacial acetic
acid to form 1,2-bis-acetamino-4,5(?)-dichlorobenzene (see below).
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Very interesting. I guess all these N-chloro amines have explosive properties, regardless the remainder attached to the nitrogen.
I'm just not clear on one thing - what is diethylene diamine? It's not diethylamine, so what is it?
Where do you get these references from? Beilstein yes, but where can you access this so far back? Are there affordable subscriber options?
By the way I found it interesting that in the patent above Ger 301, 799 (S.C.Wack) it mentioned that these alkylaminechlorides can be detonated
'especially' well with mercury fulminates and the like once they are soaked into 'Kieselgur' which is diatomaceous earth... It may be old school
(similar to the NG that used to be absorbed in Kieselgur before making NC-based dynamite) but it may be of interest.
[Edited on 25-4-2008 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by chemoleo
Very interesting. I guess all these N-chloro amines have explosive properties, regardless the remainder attached to the nitrogen.
|
Not all of them are necessarily or readily explosive, e.g. chloroguanidine deflagrates according to Beil.
| Quote: | | I'm just not clear on one thing - what is diethylene diamine? It's not diethylamine, so what is it? |
Diethylenediamine, a.k.a. piperazine (C2H4)2.(NH)2, made from ethylene chloride and alcoholic NH3, etc.
| Quote: | | Where do you get these references from? Beilstein yes, but where can you access this so far back? Are there affordable subscriber options?
|
Beilstein exists in any decent library, but you can also find a couple volumes online (e.g. Internet archive or google books). I'm not sure if there
is an online system of subscription to access of all of the volumes, possibly.
| Quote: | | By the way I found it interesting that in the patent above Ger 301, 799 (S.C.Wack) it mentioned that these alkylaminechlorides can be detonated
'especially' well with mercury fulminates and the like once they are soaked into 'Kieselgur' which is diatomaceous earth... It may be old school
(similar to the NG that used to be absorbed in Kieselgur before making NC-based dynamite) but it may be of interest. |
Certainly the case.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
I think Riedel's suggestion would have been engaged in if these N-chloroamines were not suspected genotoxins (e.g. N-chloropiperidine) and most of
them didn't hydrolyse or decompose.
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I have some doubt as to their definition of "explosive", maybe it detonates when touched with a hot wire or explodes when a glass vial containing it
is thrown into a fire its not clear. My guess is that the examples given deflagrate.
One thing that it does show though is the very low ignition points of the chloramines (particularly the secondary alkyl chloramines). Below 100
degrees C. The chloramine analogues of RDX and DPT from memory also have this low ignition point. The chloronitramine of EDNA also very low ignition
point.
1,4,5,8-Tetrachloro-1,4,5,8-tetraazadecalin (the chloramine analogue of TNAD) would be a better choice of simular structure, and more easily produced
via glyoxal + ethylenediamine. I was planning to try that one but never got around to it.
Another OTC possiblility is the dichloramine of isopropylamine, which is available as "roundup" weed killer as the glyphosate salt. The chlorinated
products of glyphosate (which is a secondary amine) should remain in solution.
AND heres reference to tetrachlorourea damnit, though I still not willing to accept that it exists so readily (not that I can read it), possibly
non-explosive or at least insensitive considering the amounts hes producing. Its from patent DE720206.
And lastly an abstract regarding the conversion of chloramines to bromamines via a bromide salt.
Zawalski, Robert; Kovacic, Peter. "Chemistry of N-halo compounds. 29. A convenient preparation of N,N-dibromoamines" Synthetic Communications (1978),
8(8), 549-62.
Abstract
Ten N,N-dibromoamines, e.g., N,N-dibromocyclohexylamine, Me2CHNBr2, Me3CCH2NBr2, Me3CNBr2, were prepd. in 60-90% yields by treatment of the resp.
N,N-dichloroamine in MeCN or MeOH at 0-5 with a water sol. bromide salt. Several attempts were made to prep. a mixed N,N-dihalo compd., e.g., by
reaction involving an excess of N,N-dichloro-.alpha.-aminoisobutyronitrile with various sources of bromide.
[Edited on 27-4-2008 by Axt]

|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by Axt
I have some doubt as to their definition of "explosive", maybe it detonates when touched with a hot wire or explodes when a glass vial containing it
is thrown into a fire its not clear. My guess is that the examples given deflagrate. |
Beilstein said chloroguanidine deflagrates in a capillary tube when heated to 150 deg. and bromoguanidine in the same instance deflagrates without
melting at 110 deg., different than explosions readily obtained from other related compounds. That doesn't tell us though if they are or aren't
detonable / explodable. They might also just be decomposable though.
| Quote: | 1,4,5,8-Tetrachloro-1,4,5,8-tetraazadecalin (the chloramine analogue of TNAD) would be a better choice of simular structure, and more easily produced
via glyoxal + ethylenediamine. I was planning to try that one but never got around to it.
Another OTC possiblility is the dichloramine of isopropylamine, which is available as "roundup" weed killer as the glyphosate salt. The chlorinated
products of glyphosate (which is a secondary amine) should remain in solution. |
There seems to be many possiblities from various nitrogenous bases and the range is wide from aromatic and aliphatic bases like the alkylamines (bases
with one nitrogen atom of the variety, C(n)H(2n + 3)N) - how about compounds from amines with a triple bond? Propargylamine,
aminoacetylene, etc. And compounds from bases with two nitrogen atoms of the variety C(n)H(2n + 4)N2), e.g. aliphatic hydrazines
(methyl or ethylhydrazine), tetramethyltetrazone, etc. must get some hellish unstable and toxic compounds. Also bases of the kind C(n)H(2n)N4 like
tetrazine, N(CH.NH.NH:CH)N, or C(n)H(2n+4)N4 like piperazyldihydrazine, NH2.N(CH2.CH2)2N.NH2, etc.
| Quote: | | AND heres reference to tetrachlorourea damnit, though I still not willing to accept that it exists so readily (not that I can read it),
|
The patent mentions that tri- and tetrachlorourea can not be prepared from urea and chlorine directly because what results is from further addition of
chlorine is NCl3. They say this can be avoided when the urea molecule is bound and protected with an additional molecule group (so salts from urea, or
nitrogen-atom substituted deriatives like monoacyl-, monoalkyl or monoarylureas) in a solubilized form and then treated with a calculated amount of
chlorine, etc.
| Quote: | | possibly non-explosive or at least insensitive considering the amounts hes producing. |
I know dichlorourea deflagrates when heated, but formed NCl3 explodes if the urea is heated in a covered or closed container, not sure about
tetrachlorourea. Dichlorourea also hydrolyzes from water to give NCl3, tetrachlorourea maybe. About stability, the patent mentions trichlorourea is
too easily decomposed, monochlorourea and a-symmetrical dichlorourea can only be stored for a short time, and say sym-dichlorourea and
sym-tetrachlorourea are stable when pure.
| Quote: | | Its from patent DE720206. |
That procedure should look something like this:
100 g of urea are - just as in example 1 - converted to urea chloride*. The solution is warmed to 30 deg., and 473 g of chlorine are passed into it.
After about half of the chlorine has been passed through, there is an evolution of gases which ceases if chlorine is further added. Due to any danger
of the possible formation of nitrogen trichloride at this stage, some chloroform is added, which can be later let off. The aqueous solution does not
give any crystals. After the evaporation and then resolubilization of the evaporation residue in distilled water, the determination of freely produced
iodine from KI, shows the formation of 205 g tetrachlorourea (62% of the theory).
* ... Example 1: 100 g urea are solubilized in a small amount of water and are converted to the chloride salt through the addition of hydrochloric
acid which contains 59 g HCl. ...
| Quote: | And lastly an abstract regarding the conversion of chloramines to bromamines via a bromide salt.
Zawalski, Robert; Kovacic, Peter. "Chemistry of N-halo compounds. 29. A convenient preparation of N,N-dibromoamines" Synthetic Communications (1978),
8(8), 549-62.
Abstract
Ten N,N-dibromoamines, e.g., N,N-dibromocyclohexylamine, Me2CHNBr2, Me3CCH2NBr2, Me3CNBr2, were prepd. in 60-90% yields by treatment of the resp.
N,N-dichloroamine in MeCN or MeOH at 0-5 with a water sol. bromide salt. Several attempts were made to prep. a mixed N,N-dihalo compd., e.g., by
reaction involving an excess of N,N-dichloro-.alpha.-aminoisobutyronitrile with various sources of bromide. |
A simple displacement then.
Note: B. [1895], 28, 1683 states the explosion product of dichloromethylamine is: CH3.NCl2 -> HCN + 2 HCl, just another reason why it likley never
found application.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | | Originally posted by Schockwave ... Naphthoquinone chloroimide. C20H10NClO3 (?). Formation. By treating a-amido-a-napthalene
with chloride of lime solution. - Bright-brown needles (from dilute acetic acid). Meltpnt.: 85 deg. Explodes at 130 deg. Barely soluble in water,
easily in alcohol, ether and acetic acid. |
Note: this should read a-amido-a-napthol chloride.
[Edited on 27-4-2008 by Schockwave]
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Schockwave
There seems to be many possiblities from various nitrogenous bases and the range is wide from aromatic and aliphatic bases ...
how about compounds from amines with a triple bond?...
|
Aromatic chloramines are particularly unstable, due to them wanting to rearange to chlorinate the benzene ring. N,N-2,4,6-pentachloroaniline is said
to be somewhat stable (Acad. Sci. Munich. Ber. (1914), 46 2728-36). Chlorination(oxidation) of the the likes of <a
href="http://www.sciencemadness.org/talk/viewthread.php?tid=5813">DAF</a> results in coupling through a azoic bridge.
Chloraminoalkynes would be hard to do, at least through direct chlorination of the amine. I would think trying to chlorinate an aminoalkyne will
result in addition across the triple bond.
An interesting explosive moiety would be -O-NCl2, giving the chloramine analogue of the nitrates. The prep of H2N-O-C2H4-O-NH2 is in PEP (2006),
31(3), 196-204. I cant view it right now but from memory I think it refers its preparation to another article which I'm sure I attached at some time
into this forum though I cant find it now. The "oxydichloramine" group is not known to scifinder.
Thanks for the translation, it seems quite odd that tetrachlorourea as the patent says is so soluble and stable. Also considering that only one
reference refers to such a simple compound :/ its hard to trust a patent.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | | Originally posted by Axt Aromatic chloramines are particularly unstable, due to them wanting to rearange to chlorinate the benzene ring.
N,N-2,4,6-pentachloroaniline is said to be somewhat stable (Acad. Sci. Munich. Ber. (1914), 46 2728-36). Chlorination(oxidation) of the the likes
of <a href="http://www.sciencemadness.org/talk/viewthread.php?tid=5813">DAF</a> results in coupling through a azoic bridge.
|
I've also found an aromatic chloroamine, N-chloro deriative of aceto-p-chloroanilide:
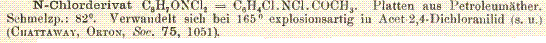
This is described as being sheets from petrol ether having a melting point of 82 deg. and decomposes explosively at 165 deg. to form
aceto-2,4-dichloroanilide.
There are also quite a few more like N-chloroderiatives of nitroanilines e.g. 4-nitro-2-chloroanilide N-chloroderiative (NO2.C6H3Cl.NCl.CO.CH3,
regular bright yellow prisms, Meltpnt. 106 deg. in B. 33, 3060), but not much beyond their pretty low melting points and some general descriptions.
| Quote: | | Chloraminoalkynes would be hard to do, at least through direct chlorination of the amine. I would think trying to chlorinate an aminoalkyne will
result in addition across the triple bond. |
Alkynes seem to survive hypochlorites, but forms highly unstable compounds.
| Quote: | | An interesting explosive moiety would be -O-NCl2, giving the chloramine analogue of the nitrates. The prep of H2N-O-C2H4-O-NH2 is in PEP (2006),
31(3), 196-204. I cant view it right now but from memory I think it refers its preparation to another article which I'm sure I attached at some time
into this forum though I cant find it now. The "oxydichloramine" group is not known to scifinder. |
The closest I've been able to find to this is trichlorooxypropylamine [OH.CH2.CH(CH2Cl)-]3N mentioned in Beil and they basically just say it has a
melting point of 92-93 deg and is made from epichlorohydrin and ammonia (B. 21 [2] 646), according to that structure it doesn't look like an analogous
-O-NCl2 though.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Cholroxysulfurpentafluoride & derivatives
| Quote: | Originally posted by Schockwave
| Quote: | Originally posted by Axt
An interesting explosive moiety would be -O-NCl2, giving the chloramine analogue of the nitrates. The prep of H2N-O-C2H4-O-NH2 is in PEP (2006),
31(3), 196-204. I cant view it right now but from memory I think it refers its preparation to another article which I'm sure I attached at some time
into this forum though I cant find it now. The "oxydichloramine" group is not known to scifinder. |
|
US 3582292
View _
http://www.google.com/patents?id=XVVlAAAAEBAJ&pg=PA1971&...
Download _
http://www.google.com/patents/pdf/ULTRA_VIOLET_RADIATION.pdf...
Some nerve agent precursors having a similar chemistry.
.
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Theres quite a few references to oxydifluoramines, though there always attached to fluorocarbons, not so great for explosive performance, well unless
you include Al in the mix. Along with the sulfur compound mentioned above the simplest one trifluoromethane derivative is described in the following.
Hale, William H., Jr.; Williamson, Stanley M. Pentafluorosulfur and trifluoromethyl oxydifluoramines. Preparations and properties. Inorg. Chem.
(1965), 4(9), 1342-6. [attached]
And CF2(ONF2)2 is detailed in US3663588. As a possible oxidant in rocket fuel.
And this, which may be of more interest though I haven't seen it.
Fokin, A. V.; Studnev, Yu. N.; Stolyarov, V. P.; Valiev, R. Sh. Organic compounds bearing a difluoroaminooxy group. Russian Chemical Bulletin
(Translation of Izvestiya Akademii Nauk, Seriya Khimicheskaya) (1999), 48(1), 131-135.
Fluorine chemistry is a bit inaccessable. The only citation given for chlorine is as the dichloronitronium ion +O-NCl2, none for its compounds.
And I'll just chuck in a couple more citations regarding the preparation of some explosive dibromamines, available online but not to me;
"Dibromamin: Alkylderivate" , Journal Monatshefte für Chemie / Chemical Monthly, Issue Volume 104, Number 6 / November, 1973, Pages 1681-1689, DOI
10.1007/BF00909655
"Dibromamin: Acylderivate", Journal Monatshefte für Chemie / Chemical Monthly, Issue Volume 104, Number 2 / March, 1973, Pages 421-432, DOI
10.1007/BF00903106
[Edited on 30-4-2008 by Axt]
Attachment: Pentafluorosulfur and trifluoromethyl oxydifluoramines. Preparations and properties.pdf (591kB)
This file has been downloaded 1089 times
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Okay, regarding halogenation of the oxyamines I've been going over stuff that I've already posted before but forgotten about. I posted it off topic in
the <a href="http://www.sciencemadness.org/talk/viewthread.php?tid=3681&page=1#pid40653">diazonium salt</a> thread. The reaction of
the oxyamines with hypobromic acid does not result in the separation of an oxydibromamine but rather (like DAF) is coupled through an azoic group
forming a hyponitrite ester. So for example CH3-O-NH2 + HOBr --> CH3-O-N=N-O-CH3.
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
I mentioned previously in this thread that iodine under ethylenediamine turned red. Heres an article that gives the preparation of the
tetraiodoethylenediamine.
<b>Nitrogen-iodine compounds. VIII. N-Iodo compounds of some aliphatic and alicyclic diamines.</b> Jander, J.; Knuth, K.;
Trommsdorff, K. U. Anorg.-Chem. Inst., Univ. Heidelberg, Heidelberg, Fed. Rep. Ger. Zeitschrift fuer Anorganische und Allgemeine Chemie
(1972), 394(3), 225-32.
Abstract
Reaction of H2NCH2CH2NH2, MeNHCH2CH2NHMe, Me2NCH2CH2NH2, or piperazine with NI3.NH3 or iodine chloride gave I2NCH2CH2NI2, IMeNCH2CH2NMeI,
Me2NCH2CH2NI2, or N,N'-diiodopiperazine, resp.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Wow, a synthesis using NI3.NH3, crazy!
|
|
|
| Pages:
1
2
3 |