| Pages:
1
2 |
Axt
National Hazard
   
Posts: 814
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Axt
I found the reason we don't hear much of the -N(NO2)2 group in explosive literature. |
Attaching another relevant article. This describes another route to N,N-dinitramines, though still requires a nitronium salt. Nitration of isocyanates
with NO2BF4/HNO3 in acetonitrile. No further info on properties, other then that they should be stored @ 0°C and handled in no greater quantities
then 0.5g, nasty.
[Edited on 9-12-2005 by Axt]
Attachment: new synthesis of alkyl n,n-dinitramines.pdf (433kB)
This file has been downloaded 1445 times
|
|
|
Axt
National Hazard
   
Posts: 814
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by chemoleo
Now that you made a putative N-chloro nitramine, do you think that might be worth reacting with NaNO2, or AgNO2? Do you have DMF, or similar solvents?
|
I did just happen along the reaction of alkyl chloramine with AgNO2 to form nitramine, so at least it can be done where:
R-NH-Cl + AgNO2 --> R-NH-NO2 + AgCl
It was in vol 8 PATR 2700, N68, under section on nitration though it doesnt provide any further information, nor references. EDIT: its in urbanski as
well, vol 3 pg. 11, though they both say AgNO3 not AgNO2, that has to be wrong. It does reference to A. BERG, Ann. Chim. [7] 3, 358 (1894). Makes you
wonder what could be produced from TCCA for example, maybe quick and dirty nitramide H2N-NO2 or triketo-RDX! C3N6O9.. hahahah, super dense, thats one
thats too easy to be true.
Anyway, I still had a small sample of EDNA that was produced back whenever, put it under microscope, picture on left is from literature, on right was
mine precipitated from water:
<center><img src="http://www.sciencemadness.org/scipics/axt/EDNA-crystals.jpg"></center>
Stick some VOD tables in too, 'cause I keep losing them. VOD is actually quite high but requires high pressure to achieve those densities. For example
PETN requires only 40000psi to obtain 1.740.
<center><table cellpadding="5" border="1" style="font-size:12px"><tr><td colspan="9"><b><center>EDNA - Measured
Velocity of Detonation</center></b></td><tr><td>Density (g/cm<sup>3</sup> </td><td>1.00</td><td>1.25</td><td>1.49</td><td>1.50</td><td>1.532</td><td>1
.562</td><td>1.663</td><td>1.71</td></tr><tr><td>VOD
(m/s)</td><td>5650</td><td>6600</td><td>7570</td><td>7580</td><td>7639</td><td>
;7750</td><td>8237</td><td>TMD</td></tr></table></center> </td><td>1.00</td><td>1.25</td><td>1.49</td><td>1.50</td><td>1.532</td><td>1
.562</td><td>1.663</td><td>1.71</td></tr><tr><td>VOD
(m/s)</td><td>5650</td><td>6600</td><td>7570</td><td>7580</td><td>7639</td><td>
;7750</td><td>8237</td><td>TMD</td></tr></table></center>
<center><table cellpadding="5" border="1" style="font-size:12px"><tr><td colspan="9"><b><center>EDNA - Loading
densities</center></b></td><tr><td>Pressure
(psi)</td><td>5000</td><td>10000</td><td>12000</td><td>15000</td><td>20000</td><t
d>40000</td></tr><tr><td>Density (g/cm<sup>3</sup> </td><td>1.28</td><td>1.39</td><td>1.41</td><td>1.44</td><td>1.49</td><td>1.
56</td></tr></table></center> </td><td>1.28</td><td>1.39</td><td>1.41</td><td>1.44</td><td>1.49</td><td>1.
56</td></tr></table></center>
[Edited on 1-5-2006 by Axt]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Nitramine from TCCA
| Quote: | Originally posted by Axt
Makes you wonder what could be produced from TCCA for example, maybe quick and dirty nitramide H2N-NO2 or triketo-RDX! C3N6O9.. hahahah, super dense,
thats one thats too easy to be true.
|
I just recently had this same idea occur to me , to react TCCA with Sodium nitrite
in dichloromethane. This merits investigation.
(CO)3(NCl)3 + 3 NaNO2 -> 3 NaCl + (CO)3(NNO2)3
Alternatively TCCA solid can be gassed with ammonia , then later ozonated.
(CO)3(NCl)3 + 6NH3 -> 3NH4Cl + (CO)3(NNH2)3 , ( cycloTricarbonylTihydrazinium ,
if it is more basic than ammonia , the only product then is the hydrochloride alone )
Ozone does not react with inorganic ammonium salts so only (CO)3(NNH2)3 can be
ozonolyzed , into the cyclo-TricarbonylTrinitramine.
Keeping with the idea of metasthesis , the sodium salt of nitromethane is itself
a priimary , reacted with TCCA instead of the nitrite could yield a useful compound.
(CO)3(NCl)3 + 3 NaCH2:NO2 -> 3 NaCl + ? ? ( cycloTricarbonylTriaminonitomethane )
This may possibly isomerize into the form on the right
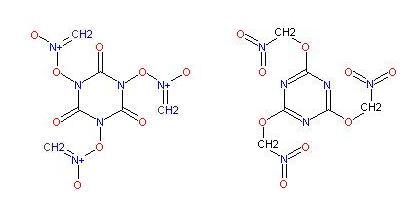
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Yet another enthusiast's viewpoint on N-Cl substitutions
N-Azidoamines and –Amides as Possible Synthons
Robert B. Steele
http://www.chemexplore.net/BookP4s.pdf
.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Triketo RDX is very dense and very powerful explosive but it is extremely unstable much more than nitramine, dinitrourea and especially sensitive to
water wich favourise hydrolysis in a autocatalytic proces...so maybe under strictly anhydrous condition and in the cold...anyway the water way might
be a good trial for nitramine generation in situ.
About the idea of Franklyn to react Na aci-nitromethane; the resulting compound will be somehow an acid anhydride and as such very prone to hydrolyse
in contact with water --> anhydrous conditions!
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Intergalactic_Captain
Hazard to Others
  
Posts: 228
Registered: 4-9-2004
Location: somewhere where i don\'t know where i am
Member Is Offline
Mood: frabjous
|
|
Has anyone had any success yet with the EG/Urea -> ethyleneurea method yet? I'd like to give this a shot over break, but if it's a no-go I've got
better things to do with my urea.
If you see me running, try to keep up.
|
|
|
maxidastier
Hazard to Others
  
Posts: 118
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
I've read this thread and wonder why this awesome topic has been shut down in the last years? Would like to try the EG/Urea -->ethylenurea as
well...
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
| Quote: | Quote: Originally posted by franklyn  |
I just recently had this same idea occur to me , to react TCCA with Sodium nitrite
in dichloromethane. This merits investigation.
(CO)3(NCl)3 + 3 NaNO2 -> 3 NaCl + (CO)3(NNO2)3
Alternatively TCCA solid can be gassed with ammonia , then later ozonated.
(CO)3(NCl)3 + 6NH3 -> 3NH4Cl + (CO)3(NNH2)3 , ( cycloTricarbonylTihydrazinium ,
if it is more basic than ammonia , the only product then is the hydrochloride alone )
|
I really do not think any of these ideas would work, as there would be several insurmountable difficulties. Firstly, substitution of the chlorine atom
in chloroalkanes with other anions is very problemetic (read about the Finkelstein reaction). Secondly, even if there existed the proper conditions,
there would be side reactions (dinitromethane, and methylene groups bridging together several cyanuric acid rings).
Reacting TCCA with NH3 would also be nearly fruitless.
The TCCA would far more readily attack the hydrazone compounds that formed than it would react with the NH3.
Even using large excesses of pressurized liquid NH3 would give low yields, because there are three, rather than one, groups on each molecule in which
optimal outcomes are sought. For example, if yields of converting "N-chloro- tetramethyl biuret", with the structure
(CH3)2NC(=O)NCl(C=O)N(CH3)2,
to the hydrazine derivitive by "gassing with ammonia" were 50%, then trying to convert TCCA to a trihydrazine derivitive would give yields of only
12.5%. Although I have wondered about reacting dimethylamine with TCCA, since the hydrazine derivitive formed may likely be protected from further
unwanted oxidation.
Originally posted by Axt
Makes you wonder what could be produced from TCCA for example, maybe quick and dirty nitramide H2N-NO2 or triketo-RDX! C3N6O9.. hahahah, super dense,
thats one thats too easy to be true.
|
You would not want to use any water (or alcohols) for the solvent, as this would only oxidize the nitrite to nitrate. Such substitution reactions can
be very problematic, as ideally both reactants must be somehow dissolved.
[Edited on 24-3-2011 by AndersHoveland]
|
|
|
maxidastier
Hazard to Others
  
Posts: 118
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
I wonder, if there is another path of producing ethylenurea that results in higher yields.
Instead of Urea as kind of a precursor you could directly use CO2 (from whatever, it is easy to obtain or produce) and Ammonia from AN or Ammonia
water
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | I wonder, if there is another path of producing ethylenurea that results in higher yields. |
There is!
Here's the patent!
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
This is off topic, but I have been recently thinking about ethylene diurea (which can be prepared by reacting
BrCH2CH2Br with excess urea dissolved in such solvents as propylene carbonate)
TNPDU can be prepared from nitration of propane diurea, so presumably TNGU could be prepared from ethylene diurea. Both TNPDU and TNGU have been well
discussed on this forum in the past. Here is the structure of TNPDU because the old link in this forum is not functioning now:
(a picture does not seem to exist anywhere online and I am having much trouble trying to post the picture or a link to the picture onto this site)
sorry to be so disorganized but there is not another easy way to do this, the picture can be found halfway down the page at http://sites.google.com/site/energeticchemical/project-defin...
http://www.chemistryjournal.info/explosives-5/w-via.html
Apparently the carbons get oxidized to "aldehyde" (although to half condensed to a urea), which then condenses to form a ring with the urea group as
soon as it forms, before it can be further oxidized.
TNPDU density: 1.93 g/cm3 velocity: 9034 m/s
described as significantly more resistant to impact
than TNGU.
[Edited on 2-4-2011 by AndersHoveland]
|
|
|
maxidastier
Hazard to Others
  
Posts: 118
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
| Quote: |
There is!
Here's the patent |
Yeah, but that we already know and I can't find an easy way to make Ethylen diamine...
So...I was talking about the gases CO2 and Ammonia.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Perhaps react acetamide with 1,2-dibromo-ethane, then after fully reacted, perform a nitration, then finally hydrolyze with a concentrated solution of
ammonium hydroxide?
1,2-dibromo-ethane can be prepared from ethylene glycol, NaBr, and concentrated sulfuric acid, although phosphoric acid is much more preferable since
there can be undesired oxidation of the hydrogen bromide. There is much relevant infotmation on this forum about such procedures (read about
preparation of iodomethane in the prepublication section), or you can read about 1-bromobutane on my site:
http://sites.google.com/site/energeticchemical/organic-precu...
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Amides aren't anywhere near as nucleophilic as amines - you won't get substitution without preparing the RCONH<sup>(-)</sup> salt first.
Also, 1,2-dibromoethane is well worth avoiding - very carcinogenic.
I weep at the sight of flaming acetic anhydride.
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Sorry for bringing up this old thread...:-)
When I was reading a bit across the net, I stumbled upon this report about the development of lead free initiator Materials: http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA438486&Locati...
My main interest was the KDNBF (or KDNP, that is now implemented as a LS replacement IIRC), but they also mention the coppersalt of EDNA as a
potential LA replacement with relatively low impact and friction sensitivity. They only give one reference from 1944 and no further information could
be found on the net. Has anyone ever made the copper salt of EDNA or has an additional reference? Is this really that energetic?
Never mind:
"This compound shows behaviour more akin to that of secondary explosives, particularlyas it neither explodes in the T of I test nor ignites in the
DSC test. In view of the greatersuitability of earlier candidates I and IV it is therefore proposed that further work on thiscompound is
curtailed."
[Edited on 28-7-2014 by nitro-genes]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
See bottom of this post _
www.sciencemadness.org/talk/viewthread.php?tid=23311#pid2745...
Following the same notion as I described here _
www.sciencemadness.org/talk/viewthread.php?tid=29259&pag...
one could experiment thus using Calcium Ethylenedinitramine and Caclium Nitrate
Ca(CH2NNO2)2 + Ca(NO3)2 => Ca2(CH2NNO2)2(NO3)2 => 2 CaO + 2 CO2 + 2 H2O + 3 N2 + O2
Structurally :
NO3 - Ca - NCH2CH2N - Ca - NO3
. . . . . . . . . N . . . . . . N
. . . . . . . . . O2 . . . . . O2
.
|
|
|
| Pages:
1
2 |