| Pages:
1
2
3
4 |
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Your favorite primary?
I am curious as to what you guys think is the best all around primary explosive for initiating less sensitive explosives? Considering ease and cost of
synthesis, sensitivity, power, toxicity, compatibility with others, etc. Exotic or common anything is fair game.
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
It's hard to beat silver nitrotetrazolate. The synthesis is a little bit involved if you have to start from scratch, but it can be done
totally using OTC chemicals.
|
|
|
froot
Hazard to Others
  
Posts: 347
Registered: 23-10-2003
Location: South Africa
Member Is Offline
Mood: refluxed
|
|
The Lead nitrate/Soduim azide/Sodium picrate azo-clathrate.
[Edited on 21-2-2008 by froot]
We salute the improvement of the human genome by honoring those who remove themselves from it.
Of necessity, this honor is generally bestowed posthumously. - www.darwinawards.com |
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
My vote is silver 5-nitrotetrazolate and compex 5-nitrotetrazole primaries. 
|
|
|
Zinc
Hazard to Others
  
Posts: 472
Registered: 10-5-2006
Member Is Offline
Mood: No Mood
|
|
Where can I find more information about silver nitrotetrazolate?
|
|
|
Sickman
Hazard to Self
 
Posts: 98
Registered: 9-5-2004
Member Is Offline
Mood: Icy and I see!
|
|
Primary Compund Explosives and Mixtures
1. 4 (Basic Lead Picrate-Lead Nitrate-Lead Azide)-11 Lead Azide] example 5 of US3431156
2. 4 (Basic Lead Picrate-Lead Nitrate-Lead Azide)-12 Lead Azide] variation
3. 4 (Basic Lead Picrate-Lead Acetate-Lead Azide)-11 Lead Azide] example 2 of US3431156
4. 4 (Basic Lead Picrate-Lead Acetate-Lead Azide)-12 Lead Azide] variation
5. Silver Azide R.D. 1336
6. Mercury Fulminate
7. Mercury Fulminate/Potassium Chlorate 90/10
8. Mercury Fulminate/Potassium Chlorate 80/20
9. Silver Acetylide
10. Silver Acetylide/Silver Nitrate Double Salt
11. Silver Acetylide/Mannitol Hexanitrate 50/50
12. Lead Nitranilate
13. Lead Nitranilate/Mannitol Hexanitrate 50/50
Well here's my developing list of "practical" primary explosives. These primaries are "practical" in the sense that they are actually capable and most
quite good at directly initiating the detonation of secondary explosives and are useful as primary charges on top of base charges in compound blasting
caps. 
These primaries are on my list of good ones for at least three reasons:
First, their precursors are practical to obtain, thus making synthesis possible. In fact I have found the most difficult precursor to obtain of those
primaries on the list is mercury for mercury fulminate. It has become the unfortunate victim of wacco environ"mental"ists who find some of mercury's
properties "evil", but even it can still be found here and there. but even it can still be found here and there.
Secondly, even though these are primary explosives there sensitivity is such that they have practical safety in handling in the sense that they don't
just detonate if you look at them. Safety precations are still essential to their handling, but these really are "safer" than many alternatives.
Thirdly, their chemical stability and storage life is such that they can be stored for a period of time, at least one year of dark, dry, and cool, and
still retain their initiatory abilities. The key to proper storage for a long life is to store in cool temperatures between 5-20*C. At such
temperatures you can expect the azides to last for decades and mercury fulminate 7-10 years (figures for storage life taken from TM 9-1300-214 Chapter
7). Silver acetylide is not as stable as mercury fulminate, but if stored in cool, dry, dark it will last more than a year and retain it's abilities
to initiate HE's. Less is known about lead nitranilate's storage life, but it is better than organic peroxides at sitting around.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
That #2 on the list , the 4/12 azo-clathrate from
Mr. Anonymous is an old favorite of mine 
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
| Quote: | Originally posted by Zinc
Where can I find more information about silver nitrotetrazolate? |
Watch 5-ATZ(5-Aminotetrazole), the nitrotetrazolate ion and friends thread.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Azo-clathrates with a twist
|
|
|
rbick
Harmless

Posts: 13
Registered: 10-7-2007
Member Is Offline
Mood: Don't Ask
|
|
This may seem somewhat lame, but let me explain first.
.1g HMTD with any amount of ETN.
A very small amount of an organic peroxide (or any explosive for that matter) will initiate basically any amount of ETN. I use this in almost all of
my blasting caps and have had great success, even when detonating the hard to initiate compositions such as PLX mixtures.
Both products can be made easily with OTC items of which are inexpensive. So while having to deal with an organic peroxide, it is a very little
amount. To initiate I use a 12v lantern battery with a switch assembly and home made e-matches.
[Edited on 22-2-2008 by rbick]
p=plenty
|
|
|
magneet
Harmless

Posts: 19
Registered: 30-12-2007
Location: on a forum somewhere
Member Is Offline
Mood: lurking mostly
|
|
Silver acetylide/nitrate.
Hi,
|
|
|
a_bab
Hazard to Others
  
Posts: 458
Registered: 15-9-2002
Member Is Offline
Mood: Angry !!!!!111111...2?!
|
|
"Mr. Anonymous is an old favorite of mine" - I *think* I know why.
Lead azide has been the best I used so far. Easy to make assuming you can get sodium azide, powerfull, less dangerous than most other "brothers".
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
| Quote: | Originally posted by Zinc
Where can I find more information about silver nitrotetrazolate? |
We did not go extensively into the properties however so:
Not sure how their formula and structure works out, I am going to assume a typo unless someone corrects me.
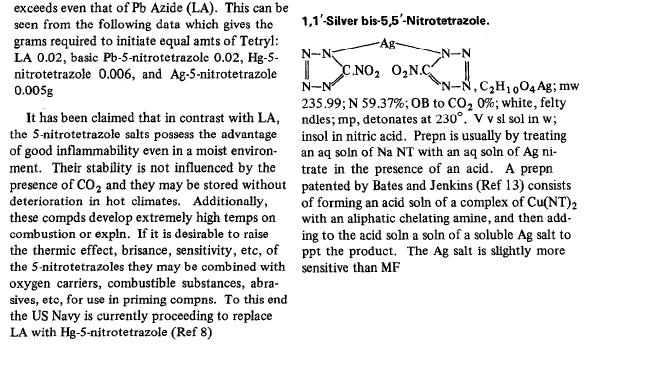
|
|
|
MadHatter
International Hazard
    
Posts: 1347
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Lead Azide
Easy to make and yes, I get sodium azide rather easily.
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
Leander
Harmless

Posts: 28
Registered: 23-2-2008
Member Is Offline
Mood: No Mood
|
|
A really good primary I once heard of was a mixture of equal parts of AgN3, Ag2C2*AgNO3 and PETN. 30 mg of this mixure is capable of detonating PETN
reliably. It is less friction-sensitive than Pb(N3)2 alone, and even less shock-sensitive than Ag2C2*AgNO3. 
There is btw one thing I don't really understand about the azo-clathrates sickman presents. Apparently you can make azo-clathrates form leadacetate
to. I always thought that azo-clathrates contain an amount of (solid) lead nitrate trapped in their cristals, in that case Pb(NO3)2 acts as an
oxidiser increasing the OB of the compound. Wouldn't the acetate only act as an intert, lowering the brisancy? Or is the solutible leadsalt (Acetate
or Nitrate) not a part of the actual compound, and just stays in solution?
[Edited on 24-2-2008 by Leander]
[Edited on 24-2-2008 by Leander]
|
|
|
Sickman
Hazard to Self
 
Posts: 98
Registered: 9-5-2004
Member Is Offline
Mood: Icy and I see!
|
|
| Quote: | Originally posted by Leander
There is btw one thing I don't really understand about the azo-clathrates sickman presents. Apparently you can make azo-clathrates form leadacetate
to. I always thought that azo-clathrates contain an amount of (solid) lead nitrate trapped in their cristals, in that case Pb(NO3)2 acts as an
oxidiser increasing the OB of the compound. Wouldn't the acetate only act as an intert, lowering the brisancy? Or is the solutible leadsalt (Acetate
or Nitrate) not a part of the actual compound, and just stays in solution?
[Edited on 24-2-2008 by Leander]
[Edited on 24-2-2008 by Leander] |
Leander,
If you took the time to read the patent US3431156, you would soon realize that any Lead salt can be used to form a "clathrate" with Lead azide. I just
like the lead nitrate and lead acetate examples, because those lead salts are easy to make or buy, and their solubility in water greatly commends them
for this purpose.
Basicaslly what you have in the "azo-clathrates" if I understand it correctly for example in:
4 (Basic Lead Picrate-Lead Nitrate-Lead Azide)-11 Lead Azide] example 5 of US3431156
is a complex crystal formation under the conditions and quantities mentioned in the patent, resulting in 4 moles of Basic Lead picrate-lead nitrate-
Lead azide complex and 11 moles of lead azide trapped within this complex crystal formation.
So to answer your question, NO! The lead acetate or lead nitrate are not left in solution they are the Lead doner and the resulting foundation for the
clathrate. Lead azide is the powerhouse of the azo-clathrates. The power output of each clathrate is for the most part extremely dependant on the
molar ratio of lead azide to crystal host.
On paper it looks like the Lead nitrate example is better than the lead acetate example, but in practice they both perform equally well at initiating
the same amount of high explosive in the same quantity, because they both contain the same amount of Lead azide. It is the case in both the lead
acetate and lead nitrate clathrates that the quantity of lead azide that they contain is what determines initiatory performance and little with wether
or not lead acetate or lead nitrate was used.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Hmmm...not to stir up a controversy , but actually the azo-clathrates which have oxidizer lead salts only are better performers than those which have
the acetate which tends to act as an inert diluent .
The two azo-clathrates for which I have a liking
are the example 5 by a modified method which gives 12 instead of 11 azides . And another unpublished variant which was tried adding a lead chlorate ,
derived from potassium chlorate and extra lead nitrate reacted in situ , seemed to extend the adsorption for azide to 16 . I didn't do any followup ,
but preliminary indications are that it is possible to exceed the limit of 13 adsorbed azide specified in the patent , at least for the case of
4(basic lead picrate-lead nitrate-lead chlorate -lead azide)-16 lead azide .
This chlorate variant was sassier than the nitrate variant ,
which was stronger than the acetate variant . The same
hierarchy of power applies to the non-azo clathrate parent compounds , which are low order detonating / igniters prior to inclusion of the azide which
transforms them into high order detonating compositions .
|
|
|
Sickman
Hazard to Self
 
Posts: 98
Registered: 9-5-2004
Member Is Offline
Mood: Icy and I see!
|
|
Rosco,
I'm going to take your word for it that the azo-clathrates with lead salts with oxidising properties are better performers, even though in my tests I
noticed no difference in application. I mean in the shock initiation of secondaries.
I should like to point out that what I said in my last post is just my opinion that the best strategy for increasing the initiatory abilites of the
clathrates is to add more moles of lead azide to the host complex which is what will really make the azo-clathrates better performers. I did get a
little careless by making things sound like OB doesn't matter at all(It does), but I think the number of moles of lead azide in the host complex is
still more definitive of performance than any other factor including oxygen balance.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Definitely the more azide content , the more powerful the azo-clathrate . But the oxidizer content also has bearing , probably with regards to the
basic lead picrate content .
A lead nitrate - lead perchlorate variant should be interesting . That oxidizer content probably has a sweet spot in its proportion similarly as do
mixtures of potassium chlorate with mercury fulminate . Getting the synergistic
energy balance / oxygen balance optimum is the point at which you wring all of the performance possible from the composition ....but only if density
isn't adversely affected .
It's really several interactive factors which will determine
which composition is hot and which is not as hot .
|
|
|
pdb
Hazard to Self
 
Posts: 90
Registered: 8-4-2004
Member Is Offline
Mood: No Mood
|
|
This thread mimics the one called "My favourite primary explosive" on the E&W forum (life is an endless repetition...).
As said on E&W, I much favor m-nitrobenzenediazonium perchlorate, in association with PETN in a caseless detonator (a pearl of primary glued on a
pill of PETN). It's a primary easy to prepare, and one of the most powerful. Diazo compounds carry a bad reputation; however, this one is considered
in the literature as one of the most stable, and I personnaly have never had any single problem the way I use it. But surely it is not a choice at
industrial scale.
|
|
|
Microtek
National Hazard
   
Posts: 872
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
If a very small detonator assembly is required I prefer silver nitrotetrazolate, otherwise I go with the azo-clathrates. However, I prefer using
azo-clathrates with only a moderate amount of azide as this produces a primary that is actually less sensitive than the secondary (I always use
compound detonators with a base charge of PETN or RDX) and requires very slight confinement in order to DDT. With RDX base charges you then get
detonators that are almost impervious to accidental ignition (shock, friction, static discharge).
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
That's interesting, could you provide more info on the clathrate you use?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
| Quote: | Originally posted by The_Davster
We did not go extensively into the properties however so:
Not sure how their formula and structure works out, I am going to assume a typo unless someone corrects me. |
Your post doesn't puzzle the others  but I do ear your tiny scream but I do ear your tiny scream  . .
In the picture you attached, they obviously made a typo between Ag and Hg in the formula first and afterwards in the tittle.
1°)Ag is usually monovalent, not bivalent, while Hg is bivalent (sometimes with inter Hg bridging making it look like monovalent ex Cl-Hg-Hg-Cl)
2°)They speak about Hg nitrotetrazolate as interesting replacement in military uses in place of LA and suddently they show the Ag salt structure...
3°)The gross formula lists 10 Hydrogen atoms instead of 10 Nitrogens
Definitely a typo.
So as a conclusion 1,1'-Mercury bis-5,5'- nitrotetrazole 
Funny to see they keep on going on further into the mistake... maybe they have fused a paragraph on the Hg and one on the Ag nitrotetrazole.
******************************
Personnally as primary,
I use Ag2C2.xHNO3 complex admixed with Ni(NH2-NH2)3(NO3)2 (mass ratio 1/2) ...unreactive towards each other, reliable from flame, stores wel for
years, non hygroscopic, easy to do and to isolate, not too shock sensitive,VOD arround 6500 m/s, lead block test arround 375 ccm/10g
But I'm sure I will find other ones I will really fall in love about.  
[Edited on 26-2-2008 by PHILOU Zrealone]
[Edited on 26-2-2008 by PHILOU Zrealone]
[Edited on 26-2-2008 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Sickman
Hazard to Self
 
Posts: 98
Registered: 9-5-2004
Member Is Offline
Mood: Icy and I see!
|
|
| Quote: | Originally posted by PHILOU Zrealone
Personnally as primary,
I use Ag2C2.xHNO3 complex admixed with Ni(NH2-NH2)3(NO3)2 (mass ratio 1/2) ...unreactive towards each other, reliable from flame, stores wel for
years, non hygroscopic, easy to do and to isolate, not too shock sensitive,VOD arround 6500 m/s, lead block test arround 375 ccm/10g.
|
What method are you using to "admix" these two? Wet? Dry? Solvent? It seems that a wet mixing process where both materials are added to H2O with
stirring and then simply filtering would be the safest.
|
|
|
pdb
Hazard to Self
 
Posts: 90
Registered: 8-4-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Microtek
If a very small detonator assembly is required I prefer silver nitrotetrazolate |
Talking about micro detonators, how does Ag nitrotetrazolate compare to AgN3 according to your experiments ?
|
|
|
| Pages:
1
2
3
4 |