Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Amine Peroxides - the others
Primary amines, when condensed with formaldehyde with H2O2 yield peroxidic products, most known is HMTD (from ammonia), and to a lesser extent TMDD
(from urea). Heres some experiments to determine the properties of some other amine peroxides and open up some more discussion on alternative amines
that could provide interesting explosive properties.
<b>Tetramethylenediperoxidedicarbamide</b>
Synthesis: 12g fertiliser grade urea was dissolved into 50ml water, then 40g 30% formaldehyde was added followed by 28g 50% peroxide and 18g 70%
nitric acid. The mixture was placed into a fridge for 3 days after which the solition had turned solid with a white precipitate. This was filtered,
flushed with water and dried.
<center><img src="http://www.sciencemadness.org/scipics/axt/TMDD.jpg"></center>
The precipitated TMDD must be completely dry to be ignited, and is very insensitive to impact. On ignition it deflagrated with the a soft orange flame
characteristic of the peroxides, but considerably slower the HMTD. <a href="http://pulse.altlist.com/images/TMDD-german.pdf">TMDD
Reference</a> (German).
<b>Ethylenetetramethylenediperoxidediamine</b>
Synthesis: 5.5g ethylenediamine was slowly injected into under the surface of 40g of 30% formaldehyde solution resulting in a very hot exotherm and
the solution turning yellow. It was placed into a freezer and brought down to -10°C. Into this was slowly injected with an eyedropper 20g of 50%
peroxide, temp had to be maintained <10°C or a violent runaway may result. On completion of the addition the solution had formed a slurry of
crystals, this was left in the fridge overnight whereby it become solid with the precipitate. I recommend diluting the formaldehyde solution before
addition of ethylenediamine.
<center><img src="http://www.sciencemadness.org/scipics/axt/HMDD.jpg"></center>
The ethylenediamine derivative is essentially HMTD minus a peroxide linkage, thus performance is lacking, although it deflagrated considerably faster
then TMDD. I never tested the impact sensitivity of HMDD. <a href="http://pulse.altlist.com/images/Caged-amine-peroxides.pdf">ETDD
Reference</a> (English).
<b>Trimethyleneperoxideazine</b>
Synthesis: 10g hydrazine sulphate was dissolved into 300ml of boiling water. Temperature was reduced to 50°C and a solution comprised of 40g 30%
formaldehyde in 20g 50% H2O2 was added to the hydrazine sulphate. A slight exotherm resulted 50->58°C and immediate precipitation of a crystaline
product. The solution was left for 30min then filtered, flushed with ether and dried.
<center><img src="http://www.sciencemadness.org/scipics/axt/TMPA.jpg"></center>
I assume trimethyeneperoxideazine takes on the dimeric structure above, thus hexamethylenediperoxidediazine. I used excess formaldehyde to try and
achieve its tetraperoxide, but on ignition it flares off at a rate between that of HMDD & TMDD, accompanied by a larger more intense flame. The
peroxide is not very sensitive to impact, even banging it with a hammer did not result in detonation. <a
href="http://pulse.altlist.com/images/hydrazine-peroxide-german.pdf">TMPA Reference</a> (German).
The reference above was translated for me by Chemoleo, below is the translation of the "important bits".
| Quote: | Anyway, regarding the trimethylazin peroxide (structure see page 496, acc. to their analyses, or I of page 492) 52 g of hydrazine sulphate is
dissolved in 1.5 l of warm 3% H2O2 (about 40-50 deg C). To this one adds a mixture of 200 ml of formalin (presumably the lab reagent percentage of 38
% w/v, just checked), and 1 l 3% H2O2.
After a short while, the crystalline peroxide precipitates. This was filtered off 1/2 hour later, washed with H2O, EtOH and ether, and dried in a
desiccator over P2O5 (I am sure drying at air will do).
Yield is 24.3 g. Anothe 4.7 g crystallised upon standing for 24 hours. Total yield 29 g, 71%. This compound is smell-less, slightly yellow, and
somewhat soluble in Chloroform. In the flame, this deflagrates rapidly with little smoke. Melting it causes explosion. Conc H2SO4 causes explosion
with the appearance of fire. It is NOT much sensitve against impact, it needs a VERY hard hammerblow to bring to explosion.
No major conclusions, except a disappointing one (and expected) which is that HMTD still surpasses the above compound in brisance, or the ethylamine
derivative (for structure see first page). |
The attachment shows three consecutive frames taken of the peroxides deflagrating. Which can also be seen in the movie <a
href="http://www.geocities.com/roguemovies8/">here</a>. Other amines may provide interesting products, hydroxylamine for example could
possibly outperform HMTD as long as the hydroxyl group is preserved. Maybe even with the correct use of solvents/temperatures and concentrations
Octamethylenetetraperoxidediazine from hydrazine could be achieved ... well maybe.
[Edited on 6-1-2008 by Axt]

|
|
|
froot
Hazard to Others
  
Posts: 347
Registered: 23-10-2003
Location: South Africa
Member Is Offline
Mood: refluxed
|
|
Any speculations on what sort of sensitivity/power the product of this procedure with ethylamine could produce? I've been thinking of trying it
but not if it could be anything like ethyl peroxide.
We salute the improvement of the human genome by honoring those who remove themselves from it.
Of necessity, this honor is generally bestowed posthumously. - www.darwinawards.com |
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
You can expect ethylamine derivative to be pretty poor, nothing like ethyl peroxide. Check the hydrazine reference for the peroxide of methylamine.
Nitrating ethanolamine -> ethanolamine dinitrate, find a solvent (maybe ethanol/ether) and try to condense with formaldehyde/peroxide could provide
something better. O2NO-CH2-CH2-N(CH2-O-O-CH2)2N-CH2-CH2-ONO2.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Why was there no citric acid needed for HMDD to form?
Does HMDD form easier than HMTD?
Ethyl peroxide- was this discussed?
What's this substance?
Still interesting, Axt, even though no high-power explosives are made this time.
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
If you pour ethylenediamine/formaldehyde/peroxide together you get pops, bangs and peroxide crystals everywhere, sadly I know this as fact  I made the mistake of assuming an acid would be needed as well. Acetic acid is used in
the reference provided, but with the concentrated solution its not needed. I made the mistake of assuming an acid would be needed as well. Acetic acid is used in
the reference provided, but with the concentrated solution its not needed.
Ethyl (hydro)peroxide is in equalibrium in a mixture of peroxide/ethanol but being soluble and only present in small concentrations, its not easily
isolated.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
When I looked up the synth. of urea, I stumbled across the Woehler type synthesis of semicarbazide, H2N-CO-NHNH2. It seems easy enough, if one has hydrazine and cyanate.
Equally, the semicarbazide can be reacted on to the bis-urea, (H2N-CO-NH)2.
I imagine both should make more interesting peroxides with CH2O and H2O2 than urea itself?!
[Edited on 13-2-2005 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
<b>Tetramethylenediperoxidebis(nitroaniline)</b>
No references for this one, but nitroaniline isn't very flammable, but the product of the synth below is, so I assume its taking on the structure
below. I dont think aniline itsef will work as it precips immediately as the aniline/formaldehyde resin.
Synthesis: 5g nitroanilne was dissolved into 200ml ethanol, into this was added 10g 30% formaldehyde and 15g 50% peroxide. A bright yellow precipitate
formed overnight which was filtered and dried.
<center><img src="http://www.sciencemadness.org/scipics/axt/tetramethylenediperoxidebis(nitroaniline).jpg"></center>
The yellow precipitate deflagrates slowly, and not very energetically with the distinct smell of burning hexamine. I also formed a precipitate from
acetaldehyde/nitroaniline/peroxide, thus probably forming a tetraethylidene derivative which hasnt been ignited, though it isn't going to do much.
Trying the higher nitroanilines would be interesting, even if they arn't going to be a practical explosive. The movie of the above was tagged onto the
end of the other <a href="http://geocities.com/roguemovies8/">amine peroxide</a> movie, though its not worth the extra 3MB download.
<center><img src="http://www.sciencemadness.org/scipics/axt/tmdpbna.jpg"></center>
Anyway, <a href="http://v3.espacenet.com/origdoc?DB=EPODOC&IDX=FR893942&F=0&QPN=FR893942">french patent 893942</a> gives some
discussion on explosive amine peroxides, focused on the derivative of tricrotonoylidenetetramine, mentions its suitable for detonators, though I'm a
sceptical considering the structure they give which is probably wrong (didn't get HMTD right either). Others are mentioned, such as derivatives of
Schiff's bases.
[Edited on 9-12-2005 by Axt]
|
|
|
Bromine
Harmless

Posts: 45
Registered: 7-9-2006
Location: Slovenija
Member Is Offline
Mood: good
|
|
What would ethyl amine + formaldhyde yield ?
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
Below is the structures assigned to the amine peroxides according to the given references. Note that a lot of these are wrong but demonstrate the
difficulty in determining the correct structure in the early years, when most of the study was done. The empirical formulas are correct however the MW
and structure vary considerably.
Ethylamine is there, however I'd think that it would form a dimeric structure due to the peroxide moiety's preference for a larger ring, thus being an
analogue to the known ammonia derivative, HMTD.
[Edited on 7-1-2008 by Axt]
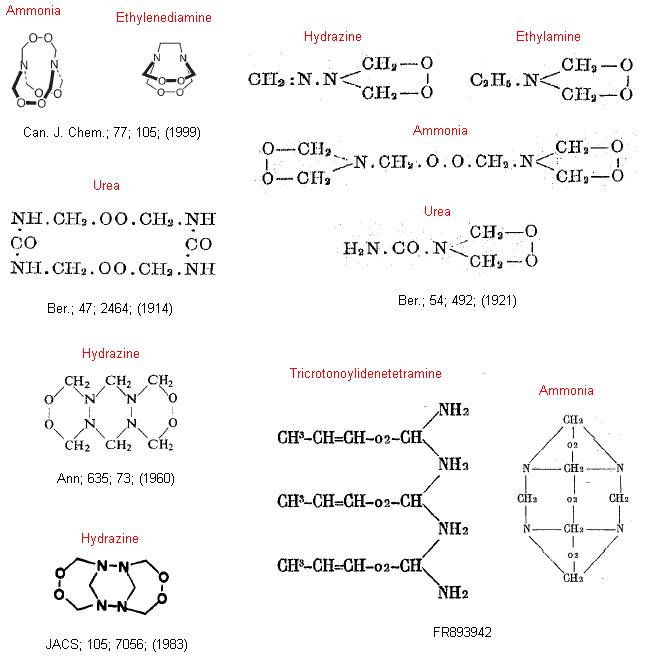
|
|
|
Bromine
Harmless

Posts: 45
Registered: 7-9-2006
Location: Slovenija
Member Is Offline
Mood: good
|
|
CH2-O-O-CH2
/ iiiiiiiiiiiiiiiiiiiiiiii \
N-C2H5 C2H5-N
\ iiiiiiiiiiiiiiiiiiiiiiii /
CH2-O-O-CH2
Would be something like that?
[Izmenjeno 6-1-2008 Bromine]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
HMTD from ammonium sulfate and formaldehyde
From https://sciencemadness.org/talk/viewthread.php?action=attach... (German)
Here they use a one-pot direction straight from ammonium sulfate and formaldehyde, perhaps someone is interested in testing this.
| Quote: | HMTD:
50 g of ammmonium sulfate (or hydrogen sulfate, it doesn't say) is dissolved in an equal mass amount of 3% commercial H2O2, brought to 55 *C and
filtered. To the warm solution 5 g of 40% formaldehyde is added. After a few moments a large amount of precipitation occurs, which is separated by
filtration after 30 minutes in the cold.
Much less time consuming than the method suggested in COPAE.
They state that the product is soluble in hot glacial acetic acid, benzene (toluene?), chloroform, and ethylacetate, where it can be recrystallised as
rhombic plates. |
In the paper they also describe peroxides of acetaldehyde, benzaldehyde and chloraldehyde.
[Edited on 20-1-2008 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
The preparation of acetaldehyde peroxide was done in accordance to the link chemoleo gave above, the quantities used were 17g H2SO4, 12g H2O and 2g
50% H2O2. This was cooled to -10°C then 1g acetaldehyde was added where temperature rose to +5°C. It was left sit in the freezer for 2 hr then at
room temperature for two days. The milky emulsion that had formed in the freezer seperated at a clear upper layer. The solution was diluted with water
whereby the oil fell to the bottom as globules, see attachment. in standing a number of days a few crystals did apperar in solution though immediately
disappeared when it was swirled.
The actual constitution of the oil isnt well given in the literature, they assigned it CH3-CH(OH)-O-O-CH(OH)-CH3 which turns into the crystalline
CH3-CH=(O-O)2=CH-CH3 on heating, though one would expect this diol derivative to undergo simular reactions to dimethylol peroxide with amines and at
least be somewhat soluble, the oil does neither. Its possibly a polymeric diperoxide? its a thin liquid at room temperature though gets quite viscous
at 0°C. Its an exceptionaly violent explosive and very sensitive, a slight tap steel on steel will result in detonation and if a small drop if
touched by the flame of a match it detonates removing the end of the said match.
Anyways to bring that back on topic the same was tried in the presence of ethylenediamine, with the same oily product as a result. This suggests that
it cant be used in analogous reactions to formaldehye, and is supported by the fact that I have never come across an amine-acetaldehyde peroxide in
the literature yet those of formaldehyde are many.
So, its probably best to ignore my claim of a a nitroaniline-acetaldehyde peroxide a few posts up as it was never tested for explosive props. I've
also since come across literature attemps at peroxides with aniline and nitroaniline with formaldehyde, though they yielded no peroxidic product (no
product was mentioned at all). So Tetramethylenediperoxidebis(nitroaniline) a few posts up looks wrong as well, which gives some explanation to the
weakly explosive product I achieved. It should be tested without the H2O2 to see if the same products are obtained.
[Edited on 15-2-2008 by Axt]

|
|
|
MWRAY236466
Harmless

Posts: 4
Registered: 12-3-2008
Member Is Offline
Mood: No Mood
|
|
I think that is not a good idea to use Nitric acid as a catalyst for the sinthesis of TMDD. What about urea nitrate ?
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
It would be interesting to try to use formamide instead of formaldehyde.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Axt  | acetaldehyde peroxide: The actual constitution of the oil isnt well given in the literature, they assigned it CH3-CH(OH)-O-O-CH(OH)-CH3 which turns
into the crystalline CH3-CH=(O-O)2=CH-CH3 on heating, Its possibly a polymeric diperoxide? its a thin liquid at room temperature though gets quite
viscous at 0°C. Its an exceptionaly violent explosive and very sensitive, a slight tap steel on steel will result in detonation and if a small drop
if touched by the flame of a match it detonates removing the end of the said match.
|
One would think it is probably structurally similar to diethyl ether peroxides. http://en.wikipedia.org/wiki/Diethyl_ether_peroxide
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
What about this one made of acetamide?

Rest In Pieces!
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Organic peroxides made from formaldehyde tend to be less chemically stable than those made from ketones. The reaction of H2O2 with CH2O can apparently
result in oxidation at ambient temperatures under some conditions. See the thread, "Producing Hydrogen by partial oxidation", http://www.sciencemadness.org/talk/viewthread.php?tid=18169
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
I have a new idea. So many ideas in my head 
TetramethyleneDiperoxideDiamine and DimethylenePeroxideAmine can be produced by hydrolysis of compounds on the left side. However, this can be done
for research of those compounds only, because it is impractical. They are probably very unstable, maybe more than HMTD.
And the materials on the left side are very interesting EM (energetic materials) themselves, I think.

Rest In Pieces!
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I do not think this would work, because the methylene groups, -CH2-, that result from the condensation of formaldehyde with ammonia have some
hydrolysis equilibrium. Those compounds you imagined would likely transiently form, but they would hydrolyse into other compounds, with the likely
expulsion of some proportion of ammonia. One of the resulting products may likely be HMTD.
The peroxide of acetamide-formaldehyde only exists because the nitrogen atoms do not have any hydrogen atoms bonded to them (which can ionize off,
precipitating hydrolysis).
[Edited on 5-1-2012 by AndersHoveland]
|
|
|