Slimz
Hazard to Others
  
Posts: 123
Registered: 18-9-2007
Member Is Offline
Mood: Inquisitive
|
|
carboxylic acid functional group
i was reading in different forum about converting a carboxylic acid functional group into an acyl chloride functional group by exposing it to thionyl
chloride.
I was wondering if someone could explain the process in a little more detail and exactly what is happening. I have tried to research this but have
reached a dead end.
If im not mistaken you should end up converting a "whater acid" to to a "whatever acid chloride" thus making that molecule receptive to amine.
I may be way off but im doing my best to understand this process.
[Edited on 3-10-2007 by Slimz]
Johnny was a chemist’s son, but now he is no more. What Johnny thought was H2O was H2SO4
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Acid chlorides can be converted back to carboxylic acids, converted to amides, converted to esters , acylated to make ketones and formation of
aldehydes by reduction.....this information can be found in any organic chemistry book , so read and read some more, there are many organic chemistry
books in the library here in the forum as well all over the net.........................solo
More acid chloride reactions....,
http://pages.towson.edu/ladon/orgrxs/carbox/clorrx.htm
[Edited on 3-10-2007 by solo]
[Edited on 3-10-2007 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Slimz
Hazard to Others
  
Posts: 123
Registered: 18-9-2007
Member Is Offline
Mood: Inquisitive
|
|
ok thanks.. the next question is where do i get thionyl chloride...or where do i get some sulfur trioxide and sulfur dichloride
BTW here is the intended reaction (this shows only the effected part of the molecule)
[Edited on 3-10-2007 by Slimz]
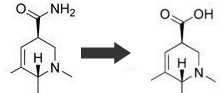
Johnny was a chemist’s son, but now he is no more. What Johnny thought was H2O was H2SO4
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Now you're off topic.....there are many threads on the subject both the synthesis and industry uses........ use the search engine .......solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Slimz
Hazard to Others
  
Posts: 123
Registered: 18-9-2007
Member Is Offline
Mood: Inquisitive
|
|
how can I be off topic, its my topic. I understand the UTFSE part.. but its not off topic..
Johnny was a chemist’s son, but now he is no more. What Johnny thought was H2O was H2SO4
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Is that supposed to be a retrosynthetic arrow or a reaction arrow? It does not fit the standards for any.
If it is retrosynthetic, then you might consider alternatives to thionyl chloride. Of course, if that is just part of the structure (you did not mark
the deleted part), nobody can help you with any advice since you did not provide the entire structure. One need to see the whole with all the
functionalities present or else the discussion is just worthless guessing. But already from what is visible one can exclude SOCl2 as the best option
since it would most likely lead to complete racemization of the chiral centre alpha to the carboxylic group and possibly also other unwanted
reactions.
PS: You really need to UTFSE by default before posting.
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
i see this guy posting crap all over the place, which he could have solved buy just reading basic oc books. this is offending.
|
|
|
jam640
Harmless

Posts: 29
Registered: 3-10-2007
Location: Rainbow Island
Member Is Offline
Mood: Fat
|
|
Looks like someone looked up lysergic acid amide on teh wikipedia and did some photoshoppin'...
Wicked skills! 
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I'm fucking tired of this too... This is not a place to get someone to tell you how to make drugs or where to buy reagents. Sometimes a particular
aspect of a drug synthesis might be discussed as a way of talking about a reaction or synthetic challenge. There's no challenge in anything you're
asking here. I think you've been warned about this. You can't learn chemistry overnight but if you were sincere in wanting to learn there would be
help available. Consider most evryone here has taken courses where hundreds of reactions on flash cards were memorized, studied and developed to
understanding. There's a certain jealousy here about preserving the intellectual integrity of the forum. When you waltz in asking how to make this
or that it brings down the whole atmosphere. You don't have to be a chemist. You just have to want to learn and try to use the resources available
to you. It's lazy and annoying to ask where to buy a particular reagent. It's intellectually enervating to ask us how to make a carboxylic acid
chloride. These are things you could have looked up. The admins have been patient to a fault with you. Man up and get a grip!
|
|
|
Maya
Hazard to Others
  
Posts: 263
Registered: 3-10-2006
Location: Mercury
Member Is Offline
Mood: molten
|
|
slimz wrote
| Quote: |
converting a carboxylic acid functional group into an acyl chloride functional group by exposing it to thionyl chloride. |
Yo, thionyl chloride will destroy the delicate backbone of that molecule slimz. Most people up to the 90's use a milder reagent like POCl3 to convert
to the acid chloride. And even better ones now with milder and more efficient yields than before.
\"Prefiero ser yo extranjero en otras patrias, a serlo en la mia\"
|
|
|
maniacscientist
Harmless

Posts: 35
Registered: 7-10-2007
Member Is Offline
Mood: No Mood
|
|
Thionylchloride is very good in doing so, you just need to take your carbonic acid and dissolve it -if neccessary-in an appropiate solvent (acid no
alcohol!) and add the stoichiometric amount of thionylchloride.
the mechanism is R-COOH + SOCl2 -> R-COCl + SO + HCl
[Edited on 18-10-2007 by maniacscientist]
|
|
|
jam640
Harmless

Posts: 29
Registered: 3-10-2007
Location: Rainbow Island
Member Is Offline
Mood: Fat
|
|
Carbonic acid is carbon dioxide dissolved in water.
And that is not a mechanism, but a chemical equation. And it is wrong.
|
|
|
maniacscientist
Harmless

Posts: 35
Registered: 7-10-2007
Member Is Offline
Mood: No Mood
|
|
I beg your pardon you´re right and english is not my mothers tongue. in my language is´t carbon acid and so is the right translation I should have
known .
The equation is right, except it should read SO2.^^ bit confused at the moment.^^:blush:
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Still that is not a mechanism. And the solvent used must be aprotic and not an acid. Besides it was already said that thionyl chloride does not work
in that idiotic above example of lysergic acid so there was no need in claiming that it "is very good in doing so".
English is not the mother tongue of many members here, including myself, but that is not an obstacle from using a spell checker and a dictionary.
|
|
|
maniacscientist
Harmless

Posts: 35
Registered: 7-10-2007
Member Is Offline
Mood: No Mood
|
|
Who was talking about LSD, this is redicoulus since this fragile molecule decomposes with minute amounts of chlorine....
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Why do you reply to threads you have not even bothered reading first? 
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
it is awful to see how dumbasses are attracted to this forum as of late.
|
|
|
Slimz
Hazard to Others
  
Posts: 123
Registered: 18-9-2007
Member Is Offline
Mood: Inquisitive
|
|
Upon further research i have found that there are indeed better more efficient ways of accomplishing what i was interested in accomplishing using
chemicals that are easier to obtain.
AKA converting to hydrazide and reacting with sodium nitrite.
[Edited on 23-10-2007 by Slimz]
Johnny was a chemist’s son, but now he is no more. What Johnny thought was H2O was H2SO4
|
|
|
Maya
Hazard to Others
  
Posts: 263
Registered: 3-10-2006
Location: Mercury
Member Is Offline
Mood: molten
|
|
That one is very old literature , actually it was in the original lit.
There are better ways still grasshopper if you look at the most recent of literature.......nice try tho,
\"Prefiero ser yo extranjero en otras patrias, a serlo en la mia\"
|
|
|
Slimz
Hazard to Others
  
Posts: 123
Registered: 18-9-2007
Member Is Offline
Mood: Inquisitive
|
|
Maya, maybe you could PM me something more specific.
Johnny was a chemist’s son, but now he is no more. What Johnny thought was H2O was H2SO4
|
|
|
Maya
Hazard to Others
  
Posts: 263
Registered: 3-10-2006
Location: Mercury
Member Is Offline
Mood: molten
|
|
nope, not gonna tell ya how to make something certain authorities frown on.
I will tell you the hydrazide you lose half your starting material right off the bat as it is converted 50% to the iso form.
maybe we'll make a chemist outta you yet, look at my previous post
\"Prefiero ser yo extranjero en otras patrias, a serlo en la mia\"
|
|
|