| Pages:
1
..
11
12
13
14
15
..
18 |
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
Thanks Der Alte. The PS I did make a long time ago - when we had that discussion about what amperage wpould be right. I thought at the time, and
thats certainly turned out to be the case in this experiment that you need 10A+ for good results. Below about 10A my cell was producing nothing.
That could also be due to the fact that to get the current that low the voltage had to be dropped to 3.6V.
The transformer you see on the photo came from a dirt-cheap welder with an adjustable current screw. Welders give 20-30V or so RMS open circuit, so I
had to wind down the secondary. I took a tap at 4V 8V and total 12V. About 2.2 V of that is dropped in the diodes. These are 35A full wave bridges
connected 4 in parallel to give a total capability of 140A. The PS is capable of about 70A before the cheap chinese transformer (they would the
secondary with Aluminium, covered with copper-coloured enamel, to make you think its copper??) starts overheating. The heatsink for the diodes you
see is actually a bit of overkill, they hardly warm to the touch in this arrangement at 70A. It would be better to mount the fan next to the
transformer. The small red winding you see on the transformer is power for a digital 200mV LCD meter which shows the current you see. I got a 100A
shunt from Jaycar across which the LCD meter is connected directly. It is switched with the switch you seeon the panel to show alternately the
voltage/current. This is measured through a low pass filter (frequency about 10Hz) so you get to see the mean value, rather than the useless peak.
[Edited on 14-9-2007 by len1]
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
| Quote: | Originally posted by 12AX7
Huh, then I'm confused about where the sodium comes from.
So the nickel anode bubbles off -- you said Castner, so this is NaOH? -- O2 and H2O, while the cathode is placed in the center of the shield and
periodically harvested?
Tim |
Yes thats absolutely right Tim. Il post some more pics as soon as I find out how to post them all at once. Len
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Great stuff len1.
I'm going to give this a go myself!
The photos are fantastic, but is there any chance you can produce a simple cross section drawing, showing how everything is arranged. Even just a
photo of a pencil sketch with the parts labelled would be a great help!
Regards, Xenoid
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
Yeah OK Ill try. Ill post a few more pictures first. I am really glad you also want to give this a go.
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
Here are more pictures of the cell

|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
I ran the cell for 150 minutes yesterday at an average current of 47A, and the resulting Na ingot can be seen in the picture.
Now the interesting thing is the current yield.
47*150*60 = 432000 Coulombs.
Now 96000 Coulombs at 100% current efficiency give 23 grams of Na. Assuming 1 gram of the weight in the pic is crud (I have tared-out the
parafin-soaked paper) that gives a yield of 23.6 grams. The percentage current yield is then
(96000/432000)*(23.6/23) = 22.8%
The maximum theoretical current yield in a castner cell is 50% due to water from the anode diffusing to the cathode (where it generates H2, so
necessary to ensure the Na doesnt catch fire). And 18% is a long-term yield quoted for industrial Castner's, so the result is not bad.
Another interesting number is the cost, which depends on the energy efficiency. As stated, and despite whats written in the literature, this 'small'
Casnter cell does not need external heating during electrolysis. So the entire energy cost is the electric power. 47A at 6.4V for 150mins assuming
80% transformer efficiency (the diode efficiency has already been accounted for in the voltage drop) is 0.94 kW-Hr. Over where I live a Kw-Hr costs
17 cents. So the cost of Na with this method is AU$6.7/Kg Na. This is VERY good.
Of course I neglected the cost of the heating current in the prior-to-electrolysis phase. Its about 12cents. So if you produce say 100gms Na in a
run, it would contribute about an extra $1.20, or $7.90 per Kg Na.
[Edited on 14-9-2007 by len1]

|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
Now that Ive run the cell several times I have established a regime under which the cell operates perfectly every time.
If anyone repeats this PLEASE WEAR ENCLOSING EYE PROTECTORS. I never approach the cell with the NaOH molten without them. Molten NaOH is one of the
best materials for destroying organic tissue. A spray bit landed on my forearm, about a 1mm sphere, and left exactly that cavity on my forearm.
If you dont follow the advice below, or your cell is constructed somewhat differently, there can be an EXPLOSION. This can eject a substantial portion
of the bath straight up the Na collector.
If this hits your Cornea - you can kiss it goodbye. And a blind experimental chemist is not a very good one.
Now to the operation.
1) The temperature of the cell must be raised GRADUALLY. In all it took about 2.5 hrs from room temperature till the contents inside the collector
were liquid. AT no stage should electrolysis begin until this has occured. Heater elements in radiative heaters are designed to operate at 800C
plus, this does not melt the quartz tube in which they are encased, but it will melt the fibreglass. I ran the coil at 50% power. This is provided
by a variable duty triac driver. Once the set temperature is reached the triac is turned off. If it is required I can post a suitable circuit. You
can basically set the heating, and then go do something else for 2.5hrs.
2) The bath melts at about 350C-360C as displayed by the thermocouple. This is due to the latent heat of NaOH melting as well as an unavoidable T
gradient between the thermocouple and the electrodes. Application of electrolysis current at this stage will lead to furious bubbling, and once the
Na starts forming small EXPLOSIONS. This is normal, most descdriptions of Castner describe this effect. However, in a large cell such an explosion
is easily contained, doesnt eject bath, or ruin the cell. For a small cell, the explosion is of the same force, but is not well contained. It will
lead to ejection of some bath. You can see the result of that in the spray present on the cover of the pot in the first picture I posted. I have
found that explosions can be entirely avoided, so NO spatter from the cell occurs at all. The procedure is as follows:
3) Once the NaOH has melted turn of the heater. Apply electrolysis current at about 3.3V but no more. The temperature starts dropping, and the cell
electrolysis residual water in the bath. It doesnt have enough voltage to electrolyse Na. As this happens the temperature starts to drop.
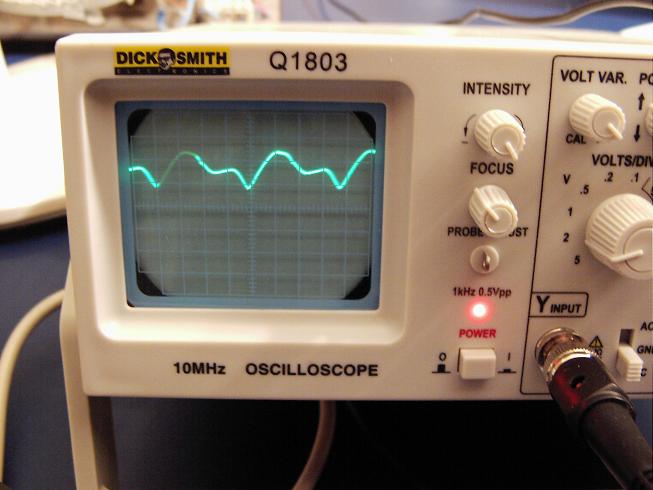
4) When it reaches 320C raise the mean voltage to 4.6V (you can see the actual variation of voltage across the cell in the scope trace below), the
current should approach 50A.
5) If the temperature rises above 325 start dropping the current by screwing in the core shunt to keep it below 330. An alteration of 1 to 2 amps
should be sufficient.
6) If the temperature reaches 330C turn the electrolysis off, until its dropped to 325 (again to avoid explosions at higher temperatures due to the
fact the the Na is more mobile in the less viscous NaOH melt).
7) If you overcompensated the other way and the temperature drops below 315 turn on the heater.
8) When starting a new run, assuming the cell is already full from the previous run, add some NaOH so bath level remains about 4cm above the cathode,
using a funnel, into the NaOH colelctor pipe.
9) Initially a brand-new ladel is a nuisance. The Na wets it perfectly and half of it stays in the ladel which is a waste. To avoid it dip the S/S
ladel into the hot NaOH bath for a minute or so, then take it out wash, and dry at 280C. This treatment will cover it with a durable black-brown
oxide cover. The Na will not stick to this.
[Edited on 14-9-2007 by len1]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Interesting pictures len. I still haven't seen where the gauze is installed, however. Is it placed between the cathode and anode as shown in the
industrial design?
Your electricity costs are interesting. If you get hard up for cash you could go into production making Na for the eBay market. There will always be
kewls wanting to throw big chunks of it into water. 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by len1
If anyone repeats this PLEASE WEAR ENCLOSING EYE PROTECTORS. I never approach the cell with the NaOH molten without them. Molten NaOH is one of the
best materials for destroying organic tissue. A spray bit landed on my forearm, about a 1mm sphere, and left exactly that cavity on my forearm.
[Edited on 14-9-2007 by len1] |
I'll say amen to that!
One of the reasons, I've never got around to building a Castner cell is my experience as a teenager many, many years ago. I knocked together a rickety
cell comprising a steel cooking dish anode and a graphite rod cathode. The NaOH was melted using a gas burner and the electrodes were connected to a
car battery. After the NaOH had melted I was lowering the cathode into the dish when it slipped and fell in!.. 
There was a bright orange flash and molten NaOH was distributed over much of my woodshed "laboratory". Fortunately the dish had also slipped sideways
and the contents were deflected away from me. I did however get several drops on the backs of my hands and arms. The scars from which I carry to this
day! I had no protective clothing or eye protection, I was very lucky.
Molten NaOH is very nasty, if you are making this cell or something similar, or carrying out manganate fusions, take appropriate precautions, as
outlined by len1.
Regards, Xenoid
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
Do you have any plans to try running the cell with potassium hydroxide?
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
I have no plans for KOH because I really have nothing I need potassium for (and not much I need sodium for either). It is harder to make due to the
higher mp of KOH, and the higher reactivity of K at these temperatures. K at 420C is not as docile as Na at 320. But you, or anyone lese is welcome
to try.
Magpie, I unfortunately can not give you pics of the gauze inside the collector due to the fact that I did not take any while the cell is being made.
You would appreciate that taking the cell apart and removing the caustic is a lot of work (the caustic forms crusts which no longer easily dissolve in
water and so the cell needs to be emptied hot) and something I intend doing until the electrolyte is due for replacement.
After having run the cell many times I thought the triac driver circuit is not really needed. That is because it turned out the optimum duty for the
element in 50% - and this can be achieved more easily with a diode half-wave rectifier in series with the winding. The diode cannot be a 1N4004, it
must be a 5 amp variety at least.
That sound a really unpleasant experience Xenoid, and something I myself have been worried about. Fortunately this cell almost completely encases the
caustic, except the Na collector, where the caustic is about 7cm below the surface. I normally put a cap on it, except when harvesting sodium. I
have a few sodium burns - the ladel spits when you wash it after harvesting - but they are small and almost healed. PS where is Codfish Island - I
grew up in NZ but dont remember a place like that.
I have run the cell many times now, and can say its very reliable, just follow the procedure I outlined. The longest I ran the cell is 7 hours on
sunday, and got 63gms Na for my trouble.
Perhaps my calculation of the cost of Na are a funny thing to do, but I have seen it mentioned that it is cheaper to buy Na that make it. The
calculations show its not so. The overhead for the cell is perhaps $80 overall (I had the old welder lying around anyway). I would not of course
ever engage in the dangerous practise of selling Na. If someone blinds himslef or someone else, you are responsible, and not just financially but
morally too. I know that some people get a joy of throwing Na into water. My kids for example - but they are young and I can understand that. Half
way through explanations of the elechtrochemical series of metals they go to me 'dad can we throw some more Na into water'.

|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Very impressive, it is interesting to see someone do the Castner cell! Pretty clean 'ingots' you are getting as well. I've had a shot before with the
CaCl2/NaCl electrolysis, but this is much less thermally intensive. Only the molten hydroxide is a pain.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
Thanks fleaker. I also tried NaCl/CaCl2 higher up in this thread, because I thought molten NaOH too dangerous, It made some Na but I couldnt get past
the pilot run. The trouble was that the melt would expand when cooled, and that would crack the ceramic cell.
The ingots of course dont look like that straight out of the cell. They are ball shaped in the form of the ladel. I coalsce them under the parafin
by pressing together. Na has a surface tension which reminds me of mercury (it also looks just like mercury when molten although of course much
lighter) so any NaOH crud which stuck to the sodium gets pushed out to the surface, from there i remove it with tweezers, and the finer stuff push to
the side.
This of course is a far from ideal way of purifying the Na. I have yet to think up of a better way. Certainly evaporating in a vacuum would be
ideal, but that would require appartus of the same level of complexity as the cell itself regards Len
|
|
|
Twospoons
International Hazard
    
Posts: 1326
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
Maybe, if you got really ambitious, you could combine the Castner cell and vacuum distillation into one. No more ladling out the molten Na - just
vacuum distill straight into a chilled receiver, giving you your pure Na in one step.
That would be one hell of an achievement.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I frequently purify Na and K from their crusts by putting them in oil in a test tube (bp > 100°C) and adding a few drops of isopropyl alcohol.
This causes the crust to fall off and, upon heating to the melting point of the metal, coalescence of the metal to a shiny drop.
With Na/K alloy, which I use frequently for drying Et2O and THF for use in Grignards, this is an extremely useful technique. Without it, it is
difficult to get a useful amount of alloy since once the metal has split into drops, it wont coalesce again without the IPA addition.
Of course, you'll lose a slight bit of metal to alcoholate formation, but this is negligible.
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
Thanks garage chemist, that (and your link) is extremely useful. Twospoons - youre not serious?
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Here are some pictures of this purification:
Na/K alloy preparation with use of IPA
The first pic is a piece of potassium with a lot of crust on it under petroleum, the second pic is after melting it.
The third and fourth pic are after addition of IPA! The potassium flows together by itself.
Then some sodium was added and the alloy transferred into an ampoule as seen in the next two pictures.
The last two pics are of the alloy being used to dry Ether, you can see how the stirring bar separates it into a fine suspension.
[Edited on 20-9-2007 by garage chemist]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Mmmm, alkali metal flux! 
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Twospoons
Maybe, if you got really ambitious, you could combine the Castner cell and vacuum distillation into one. No more ladling out the molten Na - just
vacuum distill straight into a chilled receiver, giving you your pure Na in one step.
That would be one hell of an achievement. |
It certainly would be, if you consider the following.
The vapour pressure of sodium at 440 C is about 1 mmHg. That's roughly 100 C higher than Len is running at. Going to take some fairly hard vacuum to
get much distilling.
And then all the time the sodium is being made, O2 and H2 are also coming off the cell. To handle those gases while maintaining the low pressure
needed, Len's going to need a bank of turbopumps that'll overheat the substation he's connected to.
And while that sodium is boiling off, it's mixed with those gases. Even if you use a twin vacuum system to keep the O2 and H2/Na streams separate,
the sodium and hydrogen may react.
|
|
|
Twospoons
International Hazard
    
Posts: 1326
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
It was only a wild "blue-sky" idea. You could use an additional boiler element where the sodium collects to push the temp to 600 C - then the vapour
pressure rises to around 10kPa. Go to 880 and its boils at atmospheric.
So its not impossible - just not easy.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
At those temperatures the sodium immediately reacts with the NaOH, forming Na2O. That distillation approach is unrealistic for a NaOH cell.
The distillation method would be more appropriate for a Downs cell that uses pure NaCl as the electrolyte! You could have the temperature high enough
that no vacuum at all would be necessary.
|
|
|
jimmyboy
Hazard to Others
  
Posts: 235
Registered: 1-3-2004
Location: Texas
Member Is Offline
Mood: No Mood
|
|
I still don't quite understand your cell -- maybe a schematic/drawing would help? -- it sounds like you are keeping the sodium metal separated from
the hydroxide melt with the mesh and fine temperature control -- but I'm not sure.. (has anyone else made one yet?) very impressive sodium ingot by
the way..
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
Drawing the cell takes quite some time. Ive already spent a lot of time making it and taking pictures. The pictures are quite detailed I believe.
If after that you still have problems ask me specific questions, and Ill talk you thru it. Most people are not serious so I want to see that some
effort has been made by someone before spending my time.
To understand where the S/S gauze goes look at the picture marked anode and Na Collector. The Na collector is the internal pipe with slots. A
cylindrical S/S mesh (bent to shape of course, fits snugly inside that and separates the inside of the collector from the anode. I have now taken the
cell apart and can say the gauze has hardly corroded with the many runs. There are no issues with cell construction I could find.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Len1 says:
| Quote: |
A cylindrical S/S mesh (bent to shape of course, fits snugly inside that and separates the inside of the collector from the anode.
|
Did you mean cathode (instead of anode)? Does it keep the sodium from migrating through the slots?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
| Quote: | Originally posted by Magpie
Len1 says:
| Quote: |
A cylindrical S/S mesh (bent to shape of course, fits snugly inside that and separates the inside of the collector from the anode.
|
Did you mean cathode (instead of anode)? Does it keep the sodium from migrating through the slots? |
Well I meant anode, but you could obviously also use cathode in that sentence and be equally correct. The slotted collector separates the spiral
copper cathode inside the collector from the cylindrical nickel anode outside. The S/S gauze fits inside the collector and stops the Na migrating
thru the slots as you say.
|
|
|
| Pages:
1
..
11
12
13
14
15
..
18 |