mhaisley
Harmless

Posts: 2
Registered: 23-9-2007
Member Is Offline
Mood: No Mood
|
|
Electrolysis of Water
Does anyone know where I can find the rates of gas production from the electrolysis of water?
|
|
|
chemkid
Hazard to Others
  
Posts: 269
Registered: 5-4-2007
Location: Suburban Hell
Member Is Offline
Mood: polarized
|
|
2H2O --> 2H2 + O2
Find the atomic mass of water. Then find the atomic mass of the oxygen and hydrogen. The atomic mass of hydrogen multiplyed by 4 (there are 2 h2
molecules each with two hydrogen) is the number of grams etc. of hydrogen yielded. Do the same for oxygen.
|
|
|
mhaisley
Harmless

Posts: 2
Registered: 23-9-2007
Member Is Offline
Mood: No Mood
|
|
Sorry, I should have been more specific in what I was looking for. I'm looking for a way to calculate the amount of gas produced as it relates to
current supplied. I believe that the distance between electrodes is also going to be important, but I'm not sure what all needs to be taken into
account to calculate this. I'm really hoping for an equation of some sort.
|
|
|
chemkid
Hazard to Others
  
Posts: 269
Registered: 5-4-2007
Location: Suburban Hell
Member Is Offline
Mood: polarized
|
|
Oh, alright then i have no idea 
Chemkid
[Edited on 23-9-2007 by chemkid]
|
|
|
Jamjar
Harmless

Posts: 18
Registered: 2-7-2007
Member Is Offline
Mood: No Mood
|
|
http://www.hydrogen.asn.au/electrolysis.htm
"Now calculate the theoretical (maximum) volume of the hydrogen produced, also in cubic meters, from the other data for the current and the time,
using "Faraday's First Law":
Vtheoretical = (R I T t) / (F p z),
where R=8.314 Joule/(mol Kelvin), I = current in amps, T is the temperature in Kelvins (273 + Celsius temperature), t = time in seconds, F = Faraday's
constant = 96485 Coulombs per mol, p = ambient pressure = about 1 x 105 pascals (one pascal = 1 Joule/meter3), z = number of "excess" electrons = 2
(for hydrogen, H2), 4 (if you're measuring oxygen production instead)."
Also see the_manufacture_of_chemicals_by_electrolysis in the Sciencemadness Library for some practical yeilds.
And http://en.wikipedia.org/wiki/Electrochemistry#Quantitative_electrolysis_.26_Faraday_Laws
First law
Faraday concluded after several experiments on electrical current in non-spontaneous process, the mass of the products yielded on the electrodes was
proportional to the value of current supplied to the cell, the length of time the current existed, and the molar mass of the substance analyzed.
In other words, the amount of a substance deposited on each electrode of an electrolytic cell is directly proportional to the quantity of electricity
passed through the cell.
Below a simplified equation of Faraday's first law:
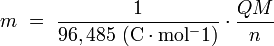
Where,
m is the mass of the substance produced at the electrode (in grams),
Q is the total electric charge that passed through the solution (in coulombs),
n is the valence number of the substance as an ion in solution (electrons per ion),
M is the molar mass of the substance (in grams per mole).
[Edited on 24-9-2007 by Jamjar]
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
For quick impromptu calculations, you can remember that a mole of electrons is about 27 ampere-hours. And of course, a mole of (ideal) gas occupies
22.4 liters at standard conditions. So if you were calculating for hydrogen you could see that you would get about a half a liter of gas by running an
amp of current 1 hour (22.4 / 27) / 2 (you divide by 2 because hydrogen gas is H2 and thus takes two moles of electrons to produce one mole of H2).
For oxygen, it takes 4 moles of electrons to produce 1 mole since oxygen has a charge of 2 and has a formula of O2. So oxygen would be produced at
half that rate. So you would need a current of approximately 2 amps to produce a liter of hydrogen per hour and 4 amps to produce a liter of oxygen
per hour.
|
|
|