octave
Harmless

Posts: 39
Registered: 4-11-2007
Member Is Offline
Mood: Working.
|
|
Benzenediols
Hello everyone! I've had a passion for chemistry ever since age 4 or 5 with Baking Soda and Acetic Acid, and I'm very much in love with organic
chemistry. So for my first post on here i have a question. Why can't the Benzenediols be synthesized from a mixture of phenol and sodium
hydroxide?Sorry if this is a really stupid question.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Welcome!
Well, why would they? What allows the hydroxide to substitute onto the ring? Replacing an H with an OH is an oxidation, but you have only base.
Suppose the H came off: you would get either a direct benzene bond (metal-carbon bonds are really, really basic, much more so than your hydroxide) or
if the OH went in place, a free H (think hydride), which is also rather basic.
Tim
|
|
|
octave
Harmless

Posts: 39
Registered: 4-11-2007
Member Is Offline
Mood: Working.
|
|
Wow i need to put my nose back in my books! Thank you for helping  . .
[Edited on 19-11-2007 by octave]
|
|
|
octave
Harmless

Posts: 39
Registered: 4-11-2007
Member Is Offline
Mood: Working.
|
|
Another question pertaining to Benzenediols. Salicylic Acid can be attained by the hydrolysis of Acetylsalicylic Acid, is there a way to further
reduce the Salicylic Acid to the Benzenediol Catechol? If so how would this be done? I've been in a fever of thinking about this even though I have
no use for Catechol yet. I know it's a useful building block in organic chemistry. Also the hydroxyl and carboxylic acid groups are at the 1,2 point
on the benzene ring. Since Catechol Is the 1,2 benzenediol that justifies my thinking.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
You can't reduce a -COOH to -OH, only to -CH2-OH. So from aspirin you could get to 2-hydroxybenyl alcohol. Two steps.
Somewhat more ambitiously you could take your salicylic acid, aminate it to 2-hydroxybenzamide, degrate that to 2-hydroxyaniline, then diazotize that
and replace the diazonium salt with -OH. Voila, your catechol. Three or four steps from aspirin, and rather a laborious way to make a cheap and
plentiful phenol.
Step 1 hydrolyze your acetylsalicylic acid
Step 2 react the salicylic acid with ammonia
Step 3 Degrade the amide via sodium hypobromite to obtain the aniline. You need NaOH and Br2. This is called a Hoffmann degredation.
Step 4 Diazotize with NaNO2 in the cold
Step 5 Hydrolyze to catechol (1,2-benzenediol)
Sic gorgeamus a los subjectatus nunc.
|
|
|
octave
Harmless

Posts: 39
Registered: 4-11-2007
Member Is Offline
Mood: Working.
|
|
Holy crap I wish I knew half as much as you do haha. Thank you for your assistance.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Sauron
...
Step 3 Degrade the amide via sodium hypobromite to obtain the aniline. You need NaOH and Br2. This is called a Hoffmann degredation.
Step 4 Diazotize with NaNO2 in the cold
Step 5 Hydrolyze to catechol (1,2-benzenediol) |
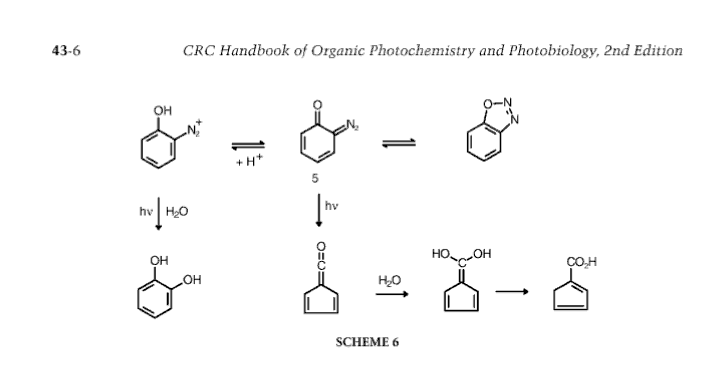
I believe that diazotising with OH, OR, and sometimes halogen in an ortho position to the amino (diazo-to-be) often gives the diazo oxide AKA
1,2,3-oxadiazole and various unexpected decomposition products of them.
Anyhow, that's one reason catechol was usually made by fusing ortho-phenolsulphonic acid or a 2-halo-phenol with KOH or NaOH (at least 50% KOH gives
higher yields)
[Edited on 25-11-2007 by not_important]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Would an oxidative decarboxylation serve here?
|
|
|