Amoled
Harmless

Posts: 31
Registered: 2-6-2017
Member Is Offline
Mood: No Mood
|
|
What group is easier to Methylate, and how to give quantitative Numbers
Good Morning
So, if I have a Compound, for example Tryptamine,
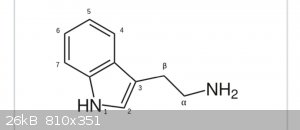
and want to Methylate at the N-position.
Is it possible to calculate how likely it is to double-methylate the primary Anime instead of the secondary amine in the indole Ring?
[Edited on 10-9-2018 by Amoled]
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
I think it depends on how you are alkylating the nitrogen. Methyl iodide? Formaldehyde/NaCNBH4? Etc, etc.
Methyl iodide would probably give you a tertiary amine. The indole nitrogen is reasonably inert to electrophilic alkylating agents.
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Interesting question. This depends on a lot of factors like choice of solvent, reagents, temperature, concentrations, etc. I don't know about
calculating, but there are probably articles on that topic that give an idea of what will most likely happen under what conditions. Selectivity is the
keyword here.
Interesting example too. I wonder why you would want to methylate tryptamine at only one of the nitrogens. Hmm...
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Loptr: I think methyl iodide would give a quaternary ammonium.
Amoled: The reactivity is determined by the nucleophilicity:
http://en.wikipedia.org/wiki/Nucleophilicity
However, the nucleophilicity varies with the acidity of the solution containing a molecule. In a strongly basic solution, the indole is deprotonated,
and when that happens it will be more nucleophilic than the amine. Under most normal conditions, the amine is much more nucleophilic than the indole,
and will be alkylated three times before the indole is alkylated.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Amoled,
Generally speaking, the hydrogen located on the 1-position of the indole ring, isn't very easily substituted.
N-Methylation or N-Dimethylation of tryptamine, by conventional means, isn't generally easily accomplished either.
Indoles are fussy, and they self-destruct, when they are displeased by reaction conditions.
So..... Alternate approaches are often employed.
Notably, N-Dimethylation via reaction with formaldehyde, and simultaneous reduction of the resultant imines with Sodium Cyanoborohydride.
Now, if you come up with a better method, let us all know.
Chemists all over the planet, have been pondering this problem for the last hundred years or so.
[Edited on 18-9-2018 by zed]
|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
Have you considered sodium cyanoborohydride in methanol?
Synthesis of DMT (and analogs) from Tryptamine
Here NaBH3CN is used in solution of methanol, GAA and formaldehyde to di-methylate the terminal nitrogen.
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quaternary ammonium compounds dealkylate on heating to form tertiary amines. Is this not the case with tetramethyltryptamine?
[Edited on 18-9-2018 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|