Volitox Ignis
Hazard to Self
 
Posts: 53
Registered: 28-1-2016
Member Is Offline
Mood: No Mood
|
|
Some Chemistry Questions
I am currently in the process of ordering some chemicals.
As of right now, I am interested in making something referred to as "Copper Cyanurate", although, I have doubts as to whether that name is correct.
It's a purple-colored compound made by reacting copper sulfate and Sodium Dichloroisocyanurate. Unfortunately, Sodium Dichloroisocyanurate is not sold
to individuals in my area.
Can Sodium Dichloroisocyanurate be made from other readily-available materials? Maybe NaOH and trichloroisocyanuric acid?
Secondly, I plan to make melamine cyanurate crystals. It consists of melamine and cyanuric acid held together by hydrogen bonds.
Cyanuric acid can be made by reacting an ammonia solution with Trichloroisocyanuric acid or by using HCl instead of ammonia.
Firstly, is making melamine cyanurate just a matter of mixing solutions of cyanuric acid and melamine and letting the solvent evaporate, or is there
something I should be aware of? Will any traces of HCl, NH3,or TCCA affect the product?
As for procuring the melamine...
I've found an article on a Russian site dedicated to chemistry
http://chemistry-chemists.com/N2_2017/ChemistryAndChemists_2...
Basically, it describes a reaction between ammonium thiocyanate and hydrogen peroxide. 3% H2O2 is sufficient. The two react to form this:
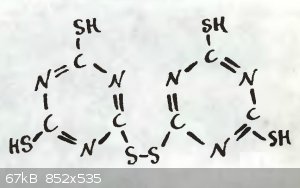
The website also claims that the substance pictured can be heated with ammonia "under pressure" to give melamine.
Does anyone here know anything about the substance? The name given is бис-(триазин-4,6-дитиол-ил-2)-дисульфида, or
bis-(triazine-4,6,-dithiolyl-2)-disulfide, and Google doesn't return any meaningful results for that.
Any information about it, including properties like melting point, solubility, or decomposition point (If it has one) would be appreciated.
Would excess peroxide react with the substance? Does anyone have any idea what pressures and temperatures would be required to make melamine from
that?Does the ammonia have to be anhydrous or will a solution work fine?
Any help is greatly appreciated.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Do NOT REACT AMMONIA WITH TCCA. Use another reducing agent such as sulfite, thiosulfate ect.
I can't find the disulfide you mention perhaps it is more complex than shown and is actually a polymer of sorts. The free thiol is known and
commercially available and according to this source can be made by trimerizing potassium thiocyanate in the presence of potassium bisulfate.
The book goes on to describe some metal salts and briefly mentions the tridisulfide compound but notes there is no experimental evidence for that
structure. I would bet it is a polymer consisting of disulfide but also sulfide groups bridging the triazines.
https://books.google.nl/books?id=VUGYsR3ZhbAC&pg=PA107&a...
[Edited on 19-7-2018 by Sigmatropic]
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Volitox:
You may be interested in the following:
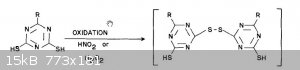
From Attachment: lthiodeisocanuricacidoughran1966.pdf (347kB)
This file has been downloaded 390 times
Borosilicate glass:
Good temperature resistance and good thermal shock resistance but finite.
For normal, standard service typically 200-230°C, for short-term (minutes) service max 400°C
Maximum thermal shock resistance is 160°C
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
"Sodium Dichloroisocyanurate is not sold to individuals in my area"
Look for solid bleach tablets at your local supermarket.
Looking for TCCA all I could find was sodium dichloroisocyanurate.
|
|
|
j_sum1
|
Thread Split
20-7-2018 at 05:09 |
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
More correctly, never react an EXCESS of a source for HOCl (including TCCA and water, sodium salt of TCCA plus acid, Cl2 and water,..) with ammonia,
amines,...
The reason is the formation of the extremely unstable yellow oily NCl3 liquid, a high explosive (with even a small amount capable of creating a
dangerously powerful explosion). The formation reaction can proceed as follows:
NH3 + HOCl --> NH2Cl + HCl
NH2Cl + HOCl --> NHCl2 + H2O
NHCl2 + HOCl --> NCl3 (separates out as a yellow oil) + H2O
Note as: NH2Cl + H2O = NH3 + HOCl, aqueous NH2Cl can be a source of HOCl to produce some NHCl2, etc. (see Equation (5) at: http://www.ecs.umass.edu/cee/reckhow/courses/ERK/slides/ERKl... ).
Given the reaction of cuprous, ferrous,..., with hypochlorous acid (a so called Fenton-type reaction), not surprisingly, such transition metal salts
can be used to attack NH2Cl in water solutions.
[Edited on 20-7-2018 by AJKOER]
|
|
|
Volitox Ignis
Hazard to Self
 
Posts: 53
Registered: 28-1-2016
Member Is Offline
Mood: No Mood
|
|
Seems like the website may have been BS. I asked someone as to what the reaction between Ammonium Thiocyanate would yield on another QnA website and
got this answer:

I checked that person's credentials. He really is who he says he is.
Anyone have other ideas on how to synthesize melamine?
| Quote: | | More correctly, never react an EXCESS of a source for HOCl (including TCCA and water, sodium salt of TCCA plus acid, Cl2 and water,..) with ammonia,
amines,... |
Well, I feel stupid for proposing using NH3 and TCCA now. I should have known better, my bad.
[Edited on 26-7-2018 by Volitox Ignis]
|
|
|