JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Cheap Alternatives to Palladium on Carbon for Heck Reaction
I have recently taken an interest in the Heck reaction, in which a carbon-carbon bond is formed between an aryl halide and a vinylic or allylic group.
I believe that the usual catalyst is palladium on carbon. Are there any readily available alternative catalysts for the Heck reaction?
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Why do you believe the catalyst is palladium on carbon? https://www.organic-chemistry.org/namedreactions/heck-reacti...
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
The first few references I read on it used palladium on carbon... that's a good site, but they tend to cite exotic applications.
Apparently, not all Pd/C catalysts work equally well... I suspect it might be possibly to achieve more reproducible results with palladium chloride.
But ideally I'd like to use a more common metal than palladium, particularly when doing early experiments where I don't really know what to expect.
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Quote: Originally posted by JJay  | The first few references I read on it used palladium on carbon... that's a good site, but they tend to cite exotic applications.
Apparently, not all Pd/C catalysts work equally well... I suspect it might be possibly to achieve more reproducible results with palladium chloride.
But ideally I'd like to use a more common metal than palladium, particularly when doing early experiments where I don't really know what to expect.
|
I think there is some literature regarding Nickel's use as the catalyst, that being said I don't know how much of it will be home chemistry friendlily
due the use of specialised ligands.
edit: here is a review which will be helpful
[Edited on 17-6-2018 by HeYBrO]
Attachment: The Heck Reaction as a Sharpening Stone of Palladium Catalysis.pdf (2.3MB)
This file has been downloaded 711 times
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
That's a pretty interesting paper.
I just ran across a paper on catalyzing Heck reactions with copper bronze in ionic liquids. I'll have to get back to this when I have the spare funds
for a couple of palladium rounds....
[Edited on 17-6-2018 by JJay]
Attachment: ol047593t.pdf (311kB)
This file has been downloaded 434 times
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. Tungsten Carbide nano-particles?
WC has been explored more as an alternative to Pt... Surface morphology of certain Crystal faces, closely resembles structure of Pt., and it has
proved a very respectable hydrogenation catalyst, for some functional groups.
I'm still having trouble getting my head around Pd. It seems radically overpriced to me.
I still remember the days when Pd was the "little brother" catalyst.
Back in those days, Pt. was around 300 per Oz, and to my recollection, Pd was around 30-40 per Oz..
About right!
Unfortunately, as Pd's list of uses has inflated, so has its price tag.
https://www.google.com/search?client=firefox-b-1&ei=584q...
As of today, it seems that Pd's spot price is actually higher than Pt's.
http://www.kitco.com/market/
One of the certain signs, that the apocalypse is looming. Bad Mojo!
https://www.poetryfoundation.org/poems/43290/the-second-comi...
[Edited on 20-6-2018 by zed]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
It's not an "alternative catalyst", but organocopper reagents act as strong Michael donors. This may be a suitable alternative process;
however, the preparation of the corresponding organocopper may be somewhat involved. It appears that the ionic liquid reaction may depend on similar
chemistry, with the copper ultimately being reduced post-rxn by the formed alkane.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I had seen something recently saying that CuCl in acetonitrile can catalyze a Heck reaction.
According to the link provided by HeYBrO, palladium salts can be used to catalyze a Heck reaction in aqueous solution. I'm not sure offhand how that
would be possible with copper....
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by clearly_not_atara  | | It appears that the ionic liquid reaction may depend on similar chemistry, with the copper ultimately being reduced post-rxn by the formed alkane.
|
I'm not sure what reaction you are referring to... in the paper I linked to above, the product is a styrene (actually an enol ester)... is there a
different paper you are looking at?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Quote: Originally posted by JJay  | Quote: Originally posted by clearly_not_atara  | | It appears that the ionic liquid reaction may depend on similar chemistry, with the copper ultimately being reduced post-rxn by the formed alkane.
|
I'm not sure what reaction you are referring to... in the paper I linked to above, the product is a styrene (actually an enol ester)... is there a
different paper you are looking at? |
I had postulated a catalytic cycle like this image
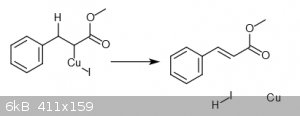
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
JJay, what product do you want to make? It might be useful for this discussion if you tell us. As for palladium that everyone seems so surprised
about, it is a common standard catalyst in academia and that is very routinely used. It is used for the Suszuki coupling, the Sonogashira coupling,
Negishi coupling and the Stille coupling (this is not an extensive list).
[Edited on 21-6-2018 by HeYBrO]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Well, palladium acetate for starters, then probably stilbene. I'd like to use cheap and reasonably environmentally friendly solvents like methanol if
possible.
I must admit that I was completely baffled at clearly_not_atara's explanation (and correction: that's probably not an enol ester), but after spending
some time studying the Heck reaction mechanism, I see what clearly_not_atara was getting at. The part I don't quite understand is why the ionic liquid
or base would be necessary with copper bronze... why didn't the authors simply allow the reaction to consume the copper bronze in a largely aqueous
medium?
[Edited on 21-6-2018 by JJay]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Quote: Originally posted by JJay  | Well, palladium acetate for starters, then probably stilbene. I'd like to use cheap and reasonably environmentally friendly solvents like methanol if
possible.
I must admit that I was completely baffled at clearly_not_atara's explanation (and correction: that's probably not an enol ester), but after spending
some time studying the Heck reaction mechanism, I see what clearly_not_atara was getting at. The part I don't quite understand is why the ionic liquid
or base would be necessary with copper bronze... why didn't the authors simply allow the reaction to consume the copper bronze in a largely aqueous
medium? |
A lot of things are unclear about that reaction. In particular, tetrabutylammonium acetate is crucial to success: the reaction produced a 10% yield
with TBAA in dimethylacetamide, but zero with an alternative base in TBAB. The reduction of copper may not happen in every solvent, and it's possible
that some exchange from an organocopper iodide to an organocopper acetate is required before that elimination proceeds. Acetate is very nucleophilic
in ILs, but when reactive cations or H-bond donors are present, the nucleophilicity of OAc- is reduced.
The catalytic cycle stabilizes copper nanoparticles in TBAB but the copper may coalesce in other solvents, reducing the surface area (and thus rxn
rate) significantly.
In water, you might see protolysis of the arylcopper ntermediate, or solvation (Cu -- O dipole-dipole interaction, eg) that shields the intermediate
from reaction, or maybe water just can't get hot enough. Ionic liquids are typically inert and TBAB especially so; in comparison water is "reactive".
See also screening acetate.
Maybe a ligated copper (0) complex could work, but maybe the ligands will interfere. Notably, high yields are only obtained with aryl iodides; aryl
bromides only react if they are electron-deficient, and even then yields are low. This suggests the lability of the C-X bond is important.
[Edited on 21-6-2018 by clearly_not_atara]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|

|
|
|