joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
The effects of temperature and concentration on reactions
How exactly does heat retards or catalyze a reaction?
Why the concentration of acids makes them more active (is this the right terminology?) towards nitration, sulfonation or whathever?
Oh, hello!  |
|
|
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
As of what I'm aware, heat makes reactions go faster because higher temperatures translate to more agitation at the molecular level, so molecules have
more chances to "bump" into each other and react. It is often said that a reaction goes twice as fast for every increase of 10 degrees celsius in
temperature. Also it could be that more molecules have the activation energy of the reaction at higher temperatures.
Edit: I also though this quote from the reaction rate page on Wikipedia might help: "Concentration: Reaction rate increases with concentration, as
described by the rate law and explained by collision theory. As reactant concentration increases, the frequency of collision increases."
[Edited on 23-4-2018 by CobaltChloride]
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Quote: Originally posted by CobaltChloride  | As of what I'm aware, heat makes reactions go faster because higher temperatures translate to more agitation at the molecular level, so molecules have
more chances to "bump" into each other and react. It is often said that a reaction goes twice as fast for every increase of 10 degrees celsius in
temperature. Also it could be that more molecules have the activation energy of the reaction at higher temperatures.
Edit: I also though this quote from the reaction rate page on Wikipedia might help: "Concentration: Reaction rate increases with concentration, as
described by the rate law and explained by collision theory. As reactant concentration increases, the frequency of collision increases."
Thank you for helping.
[Edited on 23-4-2018 by CobaltChloride] |
So in theory I could synthesize RDX by using 70% HNO3 then? It would just take a longer.
[Edited on 23/4/18 by joseph6355]
Oh, hello!  |
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Cobalt is correct;
An increase in temperature gives the molecules more energy to move around. This increased speed means increased change of collision.
An increased concentration of reactant means more moles in the reaction so more collisions. More concentrated acids are more "attacking" I guess too
to replace groups easily and they are also needed because water ruins some reactions.
Other things can be done for gaseous reaction such as increasing pressure but this has no effect on liquid reaction as liquids are quite
incompressible.
This is also, I believe, why ice baths are needed for a great many explosive synthesis such as nitrate esters where the NO2 groups readily bomd to the
oxygens. Not only because heat is released when breaking and forming the bonds, but also to slow the reaction down to avoid runaways. We all know
what happens if say a nitroglycerine synth gets too hot because of improper cooling.
On the other hand, for explosives like nitrated aromatics, the heat is needed to comvince those NO2 groups to jump on to those carbons.
Be good, otherwise be good at it 
|
|
|
sodium_stearate
Hazard to Others
  
Posts: 255
Registered: 22-4-2011
Location: guard duty at the checkpoint
Member Is Offline
Mood: No mask.
|
|
The process I use when making Edison
brown wax for phonograph cylinders takes
advantage of the increased solubility which
can be obtained by increasing the temperature.
The raw stearic acid is first melted and then brought
up to 190 C., at which time a small amount of the
lye-water with aluminum is added.
This creates a precipitate which must be cooked in
before the next addition of the lye-water solution.
This process continues over (16) of these additions
during a time span of roughly 1.75 hours.
During the last several of these additions, the reaction
starts becoming noticeably exothermic, and it has tendency
to run away if not watched very carefully.
The last few additions can only have the resulting
precipitate fully cooked in by elevating the temperature
to a full 270 C while stirring.
Once the last addition has been added and it's precipitate
fully cooked in, then the temperature is lowered
to 232 C. That temperature is never exceeded again,
and other ingredients are added to finish the batch.
The elevated temperature is required though, to take
advantage of the increased solubility of the partially
saponified stearic acid, while dissolving in the solid
precipitate of sodium and aluminum stearates.
"Opportunity is missed by most people
because it is dressed in overalls and it
looks like work" T.A. Edison
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Actually more heat means more kinetic energy for molecules, hence a higher chance to react because a higher percentage of reactant particles will have
sufficient activation energy for the reaction. It doesn’t necessarily mean that there is a higher chance of collision, especially with pure
reactants, but that there is a higher chance that a particle with enough energy to react will collide. Take the burning of paper for instance, where
cellulose chains are oxidised by oxygen gas to water and CO2. Oxygen is always hitting paper but maybe one in every hundred million collisions has
enough energy to oxidise some portion of a cellulose polymer which manifests as gradual degradation. Increase the temperature to 400-500C though, and
the majority of oxygen atoms around will have enough energy to react, so that increases the frequency of collisions in which O2 molecules have enough
kinetic energy to overcone the reaction’s activation energy. This subsequently leads to fire as the cellulose is rapidly oxidised and generates a
tremendous amount of chemical energy, maybe one in every 20 collisions or less (collisions which happen trillions of times a second I might add).
There’s a perfect graph to describe this and is applicable to ALL reactions - the Boltzmann distribution. This is what it looks like, with labels.
The X axis can be either speed or energy, since the two are related by Ek = mv2/2
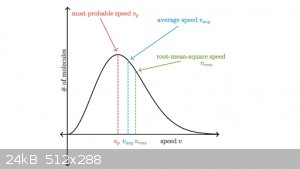
[Edited on 23-4-2018 by LearnedAmateur]
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|