joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Calculating stoichiometric amounts
I was trying to better understand the synthesis behind Trinitrophenol.
In this case I will exemplify the most common synthesis used industrially.
So, Phenol is treated with Sulfuric Acid to yield 2-Phenolsulfonic Acid and then 1-Phenol-2,4-Disulfonic Acid (or simply Phenoldisulfonic Acid) after
the equilibrium is broken.

For this, for every 1 mole of Phenol (94,01 g), 2 moles of absolute Sulfuric Acid are needed (98,07 g), thus for every 94,01 g of Phenol, we will need
106,6 ml (196,14 g) of Sulfuric Acid.
After sulfonation has been performed, the Phenol-2,4-Disulfonic Acid is then nitrated to yield 2,4,6-Trinitrophenol.

We will need 3 nitro groups attached to the phenol molecule in order to have the TNP. For each mole of nitric acid we get 1 nitro group out of it, so
3 moles of absolute Nitric Acid are needed.
Each mole of Nitric Acid weighs 63,01 g, thus, we will need 125,19 ml (189,03 g).
After nitration is performed, we have our crude desired product and also some impurities.

The problem is that 100 % Nitric Acid is not something very safe to store, and it's also somewhat expensive to synthesize than regular 68 % Nitric
Acid.
Assuming that the Nitric Acid solution is purely made by absolute H2O and Nitric Acid, it means that there is only 42,847 grams of Nitric Acid per 100
ml.
So, in order to compensate for that you will need to add more 68 % Nitric Acid Solution to achieve a whole molar equivalent of absolute Nitric Acid.
Is that correct? If that is the case, 20,16 g of Nitric Acid are missing for each molar.
Since every 100 ml of 68 % Nitric Acid gives us 42,847 grams of Nitric Acid and we need 189, 441 ml are required?
Something sounds wrong. 441 ml would be required IF 68 % Nitric Acid had a density of 1.51 g/ml, but that isn't the case, it's density is 1.41 g/ml.
Compensating for this smaller density, I get the amount of 251 ml of 68 % Nitric Acid for every molar of Phenol, thus 2,67 ml for every gram.
This amount of ml by gram sounds wrong, I'm sure that I'm doing something wrong, since powerlabs synthesis states that only 1,88 ml of 68 % Nitric
Acid should be added for every gram of phenol.
Note:
A slight excess should be added to make sure that all phenol is fully nitrated.
There is also inconclusive synthesis regarding the sulfonation. Some sources state that phenol should only be sulfonated at the para position, and
others says that it should also be sulfonated at the ortho as well as the para.
Oh, hello!  |
|
|
j_sum1
Administrator
       
Posts: 6374
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Your 68% is on a mass basis. This means you don't need to measure volume. And forget about working with densities either.
The calculation is simple.
Mass of HNO3 required ÷ 0.68 = mass of acid solution that you should measure out.
If you want say a 5% excess, multiply by 1.05.
Done.
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Quote: Originally posted by j_sum1  | Your 68% is on a mass basis. This means you don't need to measure volume. And forget about working with densities either.
The calculation is simple.
Mass of HNO3 required ÷ 0.68 = mass of acid solution that you should measure out.
If you want say a 5% excess, multiply by 1.05.
Done. |
I get 278 grams of 68 % Nitric Acid, dividing by density I get 195 ml. 2,08 ml by gram of Phenol, way off compared to PowerLabs synthesis.
Is PowerLabs wrong then?
Oh, hello!  |
|
|
j_sum1
Administrator
       
Posts: 6374
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
1 gram of phenol = 1÷94.11= 0.0106 moles
Moles of HNO3 = 0.0106 × 3 = 0.0319 moles
Mass of said HNO3 = 0.0319 × 63.01 = 2.009g
Mass of 68% acid to use = 2.009 ÷ 0.68 = 2.95g
so, roughly 3g of azeotropic acid per gram of phenol.
I don't know who PowerLabs is, but unless I have made a careless error here, the figure you quoted is incorrect.
[edit]
Converting this to mL: 2.95 ÷ 1.41 = 2.09mL per g of phenol. (Assuming your density figure of 1.41 is correct.)
[Edited on 3-4-2018 by j_sum1]
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Quote: Originally posted by j_sum1  | 1 gram of phenol = 1÷94.11= 0.0106 moles
Moles of HNO3 = 0.0106 × 3 = 0.0319 moles
Mass of said HNO3 = 0.0319 × 63.01 = 2.009g
Mass of 68% acid to use = 2.009 ÷ 0.68 = 2.95g
so, roughly 3g of azeotropic acid per gram of phenol.
I don't know who PowerLabs is, but unless I have made a careless error here, the figure you quoted is incorrect.
[edit]
Converting this to mL: 2.95 ÷ 1.41 = 2.09mL per g of phenol. (Assuming your density figure of 1.41 is correct.)
[Edited on 3-4-2018 by j_sum1] |
http://powerlabs.org/chemlabs/picric.htm
These guys.
The mistake that I made was to swap the .11 decimal to .01. Thats why my calculation is a bit off.
Oh, hello!  |
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Quote: Originally posted by joseph6355  |
There is also inconclusive synthesis regarding the sulfonation. Some sources state that phenol should only be sulfonated at the para position, and
others says that it should also be sulfonated at the ortho as well as the para. |
Anyone? My skills don't allow me to understand (yet) if Phenol should be sulfonated twice or just once.
Oh, hello!  |
|
|
greenlight
National Hazard
   
Posts: 763
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
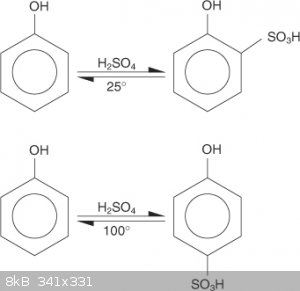
I thought that the position obtained was related to the temperature of the sulphonation and one of the products dominate (major).
When I have made TNP a long time ago, the phenol was heated with concentrated sulphuric at high temperature for a while from my memory.
According to the reaction diagram pic I have uploaded, the para position would predominate during the sulphonation of phenol to make picric acid due
to the high temperatures used.
Also found this:
"Sulphonation of benzene is a reversible reaction ie the attack of electrophile is a reversible step. So at lower temperature the attack is at ortho
positiom because it can form intramolecular hydrogen bonding and stablize the intermediate.But at higher temperatures attack occurs at para because
electrom density is higher and even if ortho attack occurs the hydrogen bond is broken very easily because of the temperature. This is one way of
looking it."
Still doesn't fully answer the question though???
[Edited on 5-4-2018 by greenlight]
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Quote: Originally posted by greenlight  | I thought that the position obtained was related to the temperature of the sulphonation and one of the products dominate (major).
When I have made TNP a long time ago, the phenol was heated with concentrated sulphuric at high temperature for a while from my memory.
According to the reaction diagram pic I have uploaded, the para position would predominate during the sulphonation of phenol to make picric acid due
to the high temperatures used.
Also found this:
"Sulphonation of benzene is a reversible reaction ie the attack of electrophile is a reversible step. So at lower temperature the attack is at ortho
positiom because it can form intramolecular hydrogen bonding and stablize the intermediate.But at higher temperatures attack occurs at para because
electrom density is higher and even if ortho attack occurs the hydrogen bond is broken very easily because of the temperature. This is one way of
looking it."
Still doesn't fully answer the question though???
[Edited on 5-4-2018 by greenlight] |
I was aware of the temperature changing the position. Actually, I was going to include this exact image of yours in my original message.
"Some sources state that phenol should only be sulfonated at the para position, and others says that it should also be sulfonated at the ortho
as well as the para."

Will attaching 2 sulfate groups change anything at all? I'm sure that there will be more byproducts, but will the TNP yield change?
That is what I can't yet understand.
[Edited on 5/4/18 by joseph6355]
Oh, hello!  |
|
|
Tsjerk
International Hazard
    
Posts: 3035
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I think you don't have much choice between the para and ortho sulfonation. The alcohol group is activating both the para and ortho position, and I
think that sulfonation of one of the positions does not change the activation of the second position much.
I guess you will always end up with a mixture of phenol/para/ortho and para+ortho sulfonated phenol.
[Edited on 5-4-2018 by Tsjerk]
|
|
|