smartgene1
Harmless

Posts: 42
Registered: 25-11-2016
Member Is Offline
Mood: No Mood
|
|
enolonium species
Hey does anyone know how to make a enolonium species from a ketone without using a hypervalent iodine reagents. Can someone please help
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Enolium? Enolate yes, but enolium? I've never heard of anything remotely similar. Is it a protonated enol? But due to their electron densities enols
are bound to protonate at carbon, not at oxygen. At which point it is simply a protonated carbonyl group.
Or do you mean alpha carbonyl cations? Such compounds are intermediates in Friedel-Crafts alkylation with alpha halo carbonyl compounds but I still
wouldn't refer to them as enolium because that resonance form would place the positive charge at the most electronegative atom (the horror, the
horror! ).
Perhaps one can arrive at an enolium compound through reaction of a ketene with a (Lewis) acid. Although I imagine the already reactive ketene will
only be more susceptible towards polymerization under such conditions.
Can you give a reference to the paper mentioning their formation with hyper valent iodine compounds? Or perhaps include astructural drawing for
clarification?
[Edited on 2-4-2018 by Sigmatropic]
|
|
|
smartgene1
Harmless

Posts: 42
Registered: 25-11-2016
Member Is Offline
Mood: No Mood
|
|
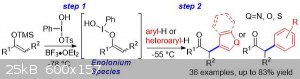
Enolonium species/iodo(III)enolates of carbonyl compounds have been suggested to be intermediates in a wide variety of hypervalent iodine induced
chemical transformations of ketones, including α‐C−O, α‐C−N, α‐C−C, and α‐carbon–halide bond formation, but they have never been
characterized. We report that these elusive umpoled enolates may be made as discrete species that are stable for several minutes at −78 °C, and
report the first spectroscopic identification of such species. It is shown that enolonium species are direct intermediates in C−O, C−N, C−Cl,
and C−C bond forming reactions. Our results open up chemical space for designing a variety of new transformations. We showcase the ability of
enolonium species to react with prenyl, crotyl, cinnamyl, and allyl silanes with absolute regioselectivity in up to 92 % yield.
https://onlinelibrary.wiley.com/doi/pdf/10.1002/anie.2016102...
I think it might be a alpha carbonyl cation but I'm not sure but it said its a umpoled enolate so it makes it a electrophile besides a nucleophile
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2788
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The question does not even make sense. An enolonium is a hypervalent iodine compound, therefore it clearly cannot be made without
iodine. Specifically, an enolonium is an enolate ester where the enol oxygen is bonded to an iodine (III) center. The name derives because the "onium"
in this case is the iodonium center.
Attached is a diagram of an enolonium reaction mechanism, for anyone who, like me, who was wondering what the fuck an enolonium is supposed to be.
|
|
|