Hilski
Hazard to Others
  
Posts: 197
Registered: 13-9-2006
Member Is Offline
Mood: No Mood
|
|
Cyanuric acid for use in preparation of cyanates
I know the production of cyanates/cyanides has already been discussed in several other threads, but I had a specific question pertaining to cyanuric
acid. I have read a few places where folks have replaced urea with cyanuric acid, and I was just wondering whether there are any disadvantages to
doing it this way. Are ther any differences in yields or purity compared to when urea is used instead of cyanuric acid?
Thanks
\"They that can give up essential liberty
to obtain a little temporary safety
deserve neither liberty nor safety. \"
- Benjamin Franklin
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I am not entirely sure this answers your question ,
or if your question applies only to cyanates and cyanides
If it is the calcium , magnesium or zinc compounds
which are relevant to your question ...
From what I have been able to glean from the very little published information on this , the route from calcium acid cyanurate is the most convenient
for a lab procedure to
a cyanate , and from there to cyanamide , or *possibly*
beyond that to a cyanide as well .
The reason cyanuric acid is preferable is that when urea is the starting material , there are additional reactions which must occur that lead to the
formation of an acid cyanurate intermediate anyway , but not so perfectly because of losses attendant to incomplete conversion , leading to an
eventual end product which is not as pure . If economy is more important than yield and purity of the end product and longer reaction times are no
concern either ....then urea is the way to go .
Using cyanuric acid simply eliminates many of the preliminary reactions and allows use of what would
otherwise be an intermediate impure conversion product in the urea process , to be used in pure form as a starting material instead , thereby
shortcutting the urea process ,
skipping over the preliminary reactions entirely , and
using a more immediate precursor which by pyrolitic decomposition alone , first forms the pure cyanate at ~300C with evolution of HOCN , and then on
heating further to the decomposition temperature of that cyanate at ~500C , evolves CO2 and gives calcium cyanamide .
A much higher temperature in the presence of added reducing agents , IIRC , is necessary for further conversion of that cyanamide to a cyanide and
this
would probably not be a complete reaction as are
the lower temperature reactions for the the precursor products , as there is not as intimate a contact for
reactions between separate components in what is a
physical mixture .....in contrast with the lower temperature
reactions for cyanate and cyanamide which are purely
decomposition reactions for a single material .
By the urea method that constraint of having a physical mixture of reactants , some solid and some molten and some gaseous intermediates ....which
never react completely as a physical mixture , is a reaction inefficiency present from the outset , and adversely affecting the purity and yield of
the product obtained at any of the three temperatures . I would qualify that conclusion
with regard to the cyanide which requires the highest
temperatures for formation , as most of my interest has
been focused on the cyanamide .
Urea can be used to accomplish the same reactions ,
but from what I can tell from the patent references especially if an adaptation to a lab scale production of cyanamide is the desire , the acid
cyanurate would
be the better precursor .
If a cyanate alone is what is desired , particularly
one of the alkali cyanates as opposed to an alkaline
earth cyanate , then perhaps the reaction of the alkali
and urea would be fine .
What end product you want in what purity and what quantity and at what price is bearable has great bearing on what strategy you will prefer .
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
If you can obtain the inexpensive reagent TCT (cyanuric chloride) then refluxing it with a large excess of acetic acid will precipitate out cyanuric
acid quantitatively.
As a side benefit you get acetyl chloride which you distill from the unreacted AcOH after filtering off the cyanuric acid cake.
This is an old method for easily preparing cyanuric acid.
The first two chlorines of TCT react rapidly, the third more slowly. So keep refluxing till no more precipitate drops out.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Here's another oldie but a goodie
Cyanuric acid synthesis doesn't get any easier or cheaper than this catalytic decomposition / conversion of urea 
This is one for the books it is so easy .
Put urea and ammonium chloride ( 1 to 0.56 molar ratio )
in a beaker and heat to melting , 15 minutes at 250C gives ~85% yield of cyanuric acid ( based on urea ) when the reaction mixture is diluted with H2O
and boiled , the cyanuric acid dihydrate crystallizing out on cooling .
The reaction should be
3(NH2CONH2) -----> (HOCN)3 + 3 NH3
This would be a fine source for dry ammonia also .
The evaporated byproduct liquid left from the manufacture of urea nitrate from urea , ammonium nitrate and HCl contains
~90% ammonium chloride and this would probably be a
convenient source for that catalyst component .
[Edited on 21-3-2007 by Rosco Bodine]
Attachment: US2527316 Cyanuric Acid via Urea and Ammonium Chloride.pdf (129kB)
This file has been downloaded 3077 times
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
http://www.freepatentsonline.com/4432959.html
If someone can get these in PDF form that would be great, they stopped pdf availability for non registered users.
I made sodium cyanurate a while back as an intermediate to calcium cyanurate, the dissolving of cyanuric acid was very annoying. The patent above has
a dry process for this. I wonder if calcium carbonate could be substituted in for sodium carbonate, giving calcium cynaurate and the entire thing hit
with high temperatures giving calcium cyanamide in a one step process?
[Edited on 7-4-2007 by The_Davster]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Registering for freepatentsonline is free and anonymous, all they want is an email address (which can be hotmail or hushmail etc.) and a password. No
name no fee no street address.
I went through the hassle of adding a TIFF viewer to my browser so I can use the USPTO website engine, as even for reg.users, freepatentsonline seems
to have dropped older patents, I could not get one from 1932 to come up at all but got it easily from USPTO.
You need to be careful which free TIFF appliance you install, as some simply don't work or even cause hangs. I assume this is ideosyncratic to
OS/browser version/config. I run XP Pro SP2 so maybe first one I tried simply was way out of date.
Here's your pdf from freepatentsonline.
Don't tell certain members you use that site or they will accuse you of using a crap site. I have used it for years and find it fast and convenient as
long as I have the pat.number.
[Edited on 8-4-2007 by Sauron]
Attachment: 4432959[2].pdf (102kB)
This file has been downloaded 1114 times
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
One pot process for urea and CaCO3 to calcium cyanamide:
http://v3.espacenet.com/textdoc?DB=EPODOC&IDX=US5753199&...
(The pot needs to be a rotary kiln)
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Thanks but this thread is about calcium cyanurate not cyanamide.
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
Just responding to the Davester. And the reaction goes via the cyanurate and cyanate. 
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
It useful if you could stop it there, but that does not seem likely at first glance does it?
|
|
|
Maya
Hazard to Others
  
Posts: 263
Registered: 3-10-2006
Location: Mercury
Member Is Offline
Mood: molten
|
|
I've found pool stores carry buckets of cyanuric acid
\"Prefiero ser yo extranjero en otras patrias, a serlo en la mia\"
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
That's odd. TCCA I can understand, but what are they using cyanuric acid for?
Maybe bringing an overchlorinated pool back to balance?
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Sauron
That's odd. TCCA I can understand, but what are they using cyanuric acid for?
Maybe bringing an overchlorinated pool back to balance? |
It's sold as a "chlorine stabiliser", I'm assuming that there's reversible chloramine formation involved. It is a handy source of cyanuric as so far
it's resisted the trend to diluting it with half a dozen other things.
http://www.aquachek.com/PublicPopups.asp?action=displayparam...
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Yeah, TCCA rearranges to cyanuric acid when it gives up its chlorines and reverts to TCCA in the presence of chlorine so I think we both surmise
correctly.
Too bad we can't get from cyanuric acid to TCT so easily. (It can be done but it takes powerful chlorinating agents like oxalyl chloride to do it and
since we would prefer to make oxalyl chloride from TCT...no point. (And of course this is not how TCT is made industrially, which is a process totally
unsuitable for the lab unless you fancy working with neat HCN a lot. TCT is trimer of cyanogen chloride.)
|
|
|
Maya
Hazard to Others
  
Posts: 263
Registered: 3-10-2006
Location: Mercury
Member Is Offline
Mood: molten
|
|
it is a chlorine stabilizer
\"Prefiero ser yo extranjero en otras patrias, a serlo en la mia\"
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
My kilo of each cyanuric acid, and TCCA says both are available.
I believe it is used to stabilize the chlorine when you use Ca(OCl)2 chlorinator, but if you are doing that I would not know why you would not just
use TCCA? Oh well, not like I am complaining...one more chemical around.
|
|
|
tupence_hapeny
Hazard to Others
  
Posts: 131
Registered: 25-3-2007
Member Is Offline
Mood: continuing respiration (touch wood)
|
|
It is the most overrepresented product in the pool supply stores here.
Here is the patent you wanted
And as for thin ice, nnnaaaahhhh, won't say it
[Edited on 10-4-2007 by tupence_hapeny]
Attachment: Process for Producing Sodium Cyanurate (US Patent 4432959).pdf (102kB)
This file has been downloaded 1511 times
We are all the sum of our experiences, and our reactions to the same
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Quote: Originally posted by Rosco Bodine  | Cyanuric acid synthesis doesn't get any easier or cheaper than this catalytic decomposition / conversion of urea 
This is one for the books it is so easy .
Put urea and ammonium chloride ( 1 to 0.56 molar ratio )
in a beaker and heat to melting , 15 minutes at 250C gives ~85% yield of cyanuric acid ( based on urea ) when the reaction mixture is diluted with H2O
and boiled , the cyanuric acid dihydrate crystallizing out on cooling .
The reaction should be
3(NH2CONH2) -----> (HOCN)3 + 3 NH3
This would be a fine source for dry ammonia also .
The evaporated byproduct liquid left from the manufacture of urea nitrate from urea , ammonium nitrate and HCl contains
~90% ammonium chloride and this would probably be a
convenient source for that catalyst component .
[Edited on 21-3-2007 by Rosco Bodine] |
Where I live the pool stores don’t carry cyanuric acid, only TCCA, so I decided to make my own.
I always tried that procedure, but my product was always contaminated (Ive used small mild steel food cans as crucibles).. The molten urea/NH4Cl
apparently leached some of the iron and the result was a red stuff.. (recrystallization didnt work to eliminate the colour).
Some weeks ago, I tried the procedure again, but using some basic 'professional' equipment..
First time, 20.4g farm grade urea and 10.0g technical grade NH4Cl were fused in a beaker (in outdoors) for some minutes.. The fused mass was
pulverized and recrystallized. Result was 4.2g of white crystalline Cyanuric Acid (CA).. Which is about ~29% of theory.. I believe this yield was low
because of small amounts of reactants (and so the acumulated loss in every step is substancial) and also I recrystallized the CA (the patent yield is
high because they only wash the product to remove NH4Cl, they didnt recrystallize the CA, IIRC).
Second time I took some photos and videos of the process..
60.0g urea and 30.8g NH4Cl were weighted and mixed:

Then the mix was transferred to a 600mL beaker, this on a electric heating panel in the outdoors.

Then the panel was set on and the mix was fused and occasionally stirred with a piece of glass rod (the spoon was used to remove crusts of solids from
the rod):




Video:
<object width="480" height="385"><param name="movie"
value="http://www.youtube.com/v/wlnc0tqRkFk?fs=1&hl=pt_BR"></param><param name="allowFullScreen"
value="true"></param><param name="allowscriptaccess" value="always"></param><embed
src="http://www.youtube.com/v/wlnc0tqRkFk?fs=1&hl=pt_BR" type="application/x-shockwave-flash" allowscriptaccess="always"
allowfullscreen="true" width="480" height="385"></embed></object>
When the ‘point’ was hit (smaller amounts of NH3 coming out of the mix) I turned off the panel and transferred the beaker to another place in the
backyard and with the rod/spoon, crushed the material still hot (and still soft… After cooling, this mass will be hard as a rock and removing it
from beaker is just PITA… So I crushed a little while still hot).

BTW, this is a good way to dry ammonia gas, but the down side is that your NH3 may be contaminated with tiny solid NH4Cl particles..
The result 68.6g of mixed CA/urea/NH4Cl/byproducts:

But some of it was adhered to beaker, even though I tried to use the brute force to remove it.. But I removed most of it later..

Lunch time! So I put again the weighted solids in the beaker and a sheet of plastic covering it (to protect it against moisture)..
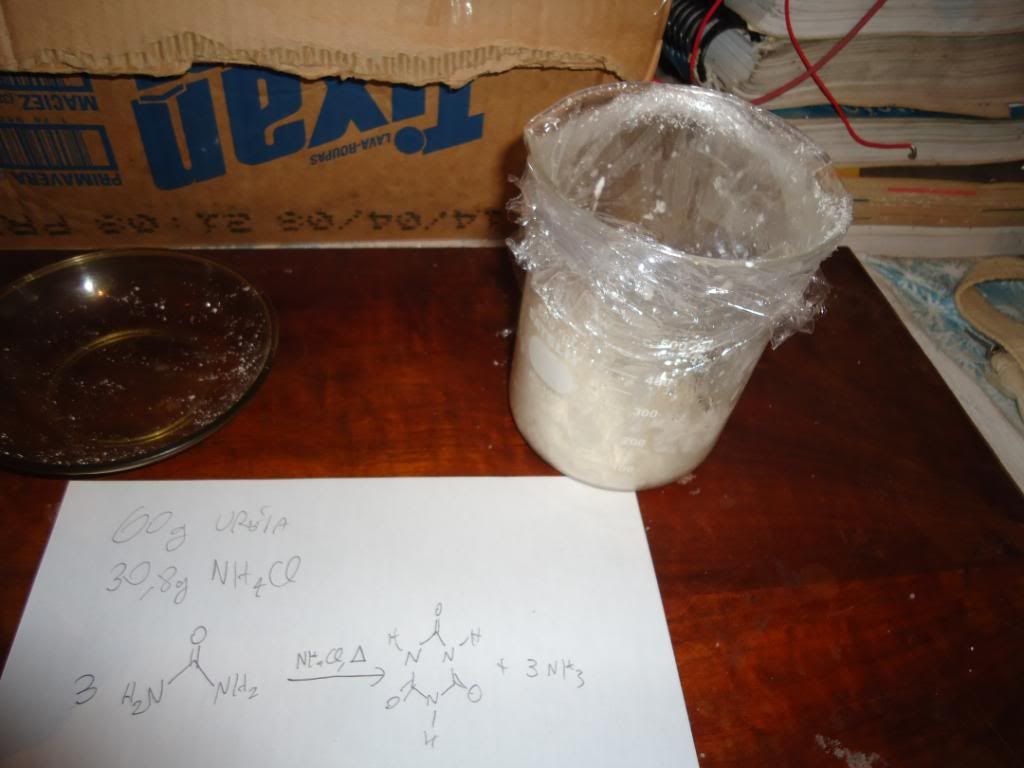
After lunch I decided to pulverize better the solids, to facilitate all the subsequent operations. Ive used a piece of PVC pipe and a paper foil to do
this job.. Portions of the solids at time were comminuted and transferred to a larger beaker..


100mL of tap water were added to the first beaker, and with the spoon under the water, I tried to remove the small bits of solids adhered to the
beaker:

The solution in the first beaker was transferred to the larger beaker with the solids, and the more 100mL of tap water was added to the smaller beaker
and the process repeated..

(The solution in the larger beaker was filtered while the solution in the first was transferred to second)
These water additions were made to remove unreacted urea, NH4Cl and other soluble things (CA solubility in RT water is low). Then two portions each
of 20mL of water were used to remove the last residues in the two beakers and to centralize the white mass in the filter. The filter was carefully
squeezed with hands, to remove interstitial liquid.







The urea/NH4Cl solution can be recycled again and again in another CA batches, but I discarded it..

The compact snow white solid was transferred again to the 1L beaker and the filter paper put above it in the way show in the next pic. Then tap
water was gradually added to it in such way to remove all the adhered solids (using also the spoon).


Then completed to ~1L level.

the compact mass was broken in the water with a glass tube.. And then this set was placed in the heating pan..



The funnel/filter was ready…

Video:
<object width="480" height="385"><param name="movie"
value="http://www.youtube.com/v/mGd8tZAMXs8?fs=1&hl=pt_BR"></param><param name="allowFullScreen"
value="true"></param><param name="allowscriptaccess" value="always"></param><embed
src="http://www.youtube.com/v/mGd8tZAMXs8?fs=1&hl=pt_BR" type="application/x-shockwave-flash" allowscriptaccess="always"
allowfullscreen="true" width="480" height="385"></embed></object>
The solution was then gradually added to the filter.. After the first passage, the PET bottle was transferred to a small plastic bowl with water (this
was made to avoid PET deformation due to hot solution)..


Then the solution was left to cool down and then cooled further with an ice-water bath.. Some crystals were in the bottom..


The mass was filtered and the ‘cake’ was washed with a little bit of water and squeezed..


The filter paper was the carefully open and this was transferred to my improvised ‘greenhouse’ (I think I forgot the correct name..ahh) under a
100W incandescent lamp..



Video:
<object width="480" height="385"><param name="movie"
value="http://www.youtube.com/v/4Vw4iHSofUs?fs=1&hl=pt_BR"></param><param name="allowFullScreen"
value="true"></param><param name="allowscriptaccess" value="always"></param><embed
src="http://www.youtube.com/v/4Vw4iHSofUs?fs=1&hl=pt_BR" type="application/x-shockwave-flash" allowscriptaccess="always"
allowfullscreen="true" width="480" height="385"></embed></object>
After dried, the material was weighted: 17.4g (~40% yield.. )


I was curious with the crystals, some of it were apparently acicular to naked eye, but other crystals not.. Then I tried to look with a
‘sci-toy’ microscope that I acquired when kid..


100x:

http://i242.photobucket.com/albums/ff176/tnitrato/QUIMICA/Cyanuric%20Acid%20-%20Acido%20Cianurico/DSC00253.jpg[/img]
200x:
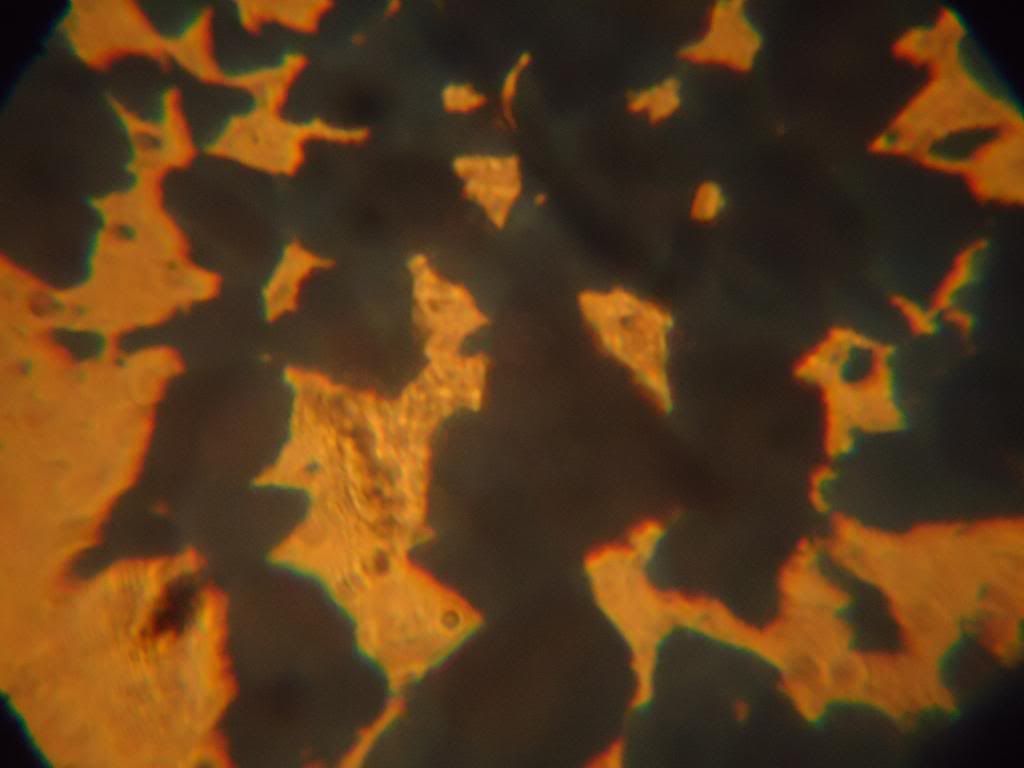
300x:
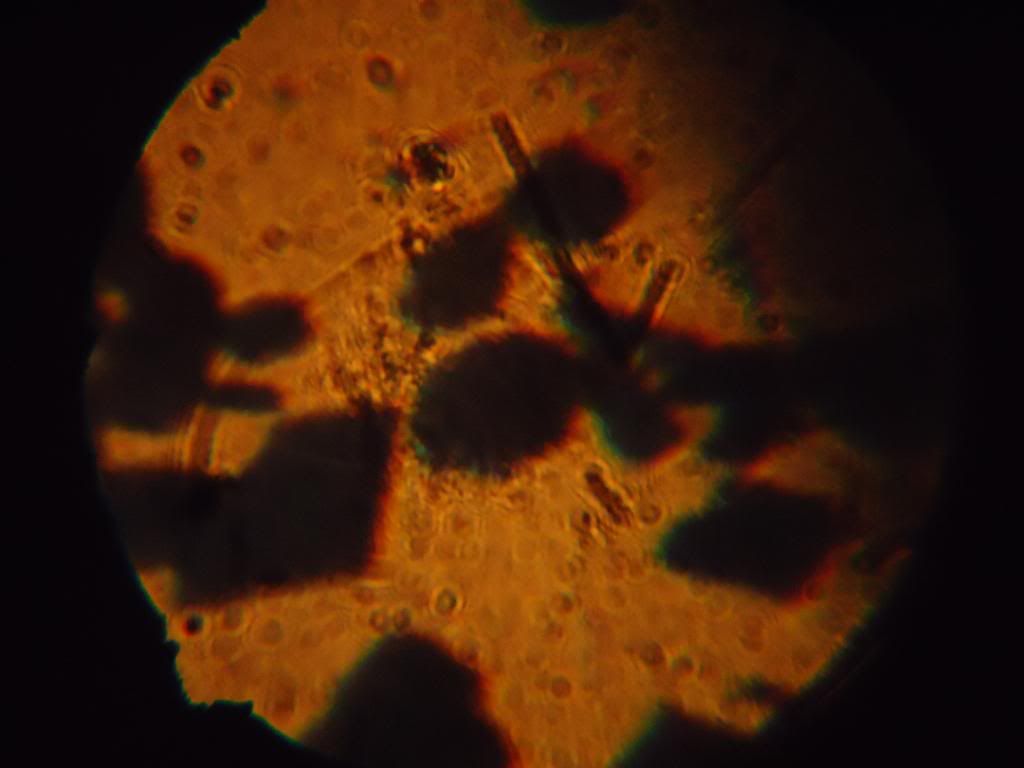
Not very conclusive.. But it was my fault because I didn’t let the crystals grow perfectly: I stirred and shake constantly the warm solution, just
to cool down faster and I get my CA…
Cyanamide, Guanidine, cyanates, cyanides and a plethora of substances are now waiting me!
Woo Hoo!!!
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Nice going Aqua.
When you finish the initial reaction if you can pour the melt directly into a mortar it will be much easier than digging rocks out of your beaker.
This is the technique used when making KOCN from urea/K2CO3 and it works quite well. If you swirl the melt so it hardens as a thin layer on the wall
of the mortar it's even better.
Your English is quite good.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Thanks for the tips, Magpie! My "mortar" is actually a cheap plastic mortar, but I guess it should work..
Thats my first experiment using real labware (the geology lab of my university have recently discarded some glassware - some with cracks and others in
perfect conditions - and then I grabed it before the garbage can).
Would not be dangerous to the beaker (crack/break) swirl/pour of this kind of melt?
BTW, what you made with your KOCN? KCN?
Thanks!
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I would not pour the melt onto plastic.
It is not a problem for the beaker as you just pour the melt directly into the mortar, then swirl until it solidifies.
I plan to make some semicarbazide. You can also make urea to repeat (approximately) Wohler's historically important synthesis.
The synthesis I use is that shown in Inorganic Synthesis. Atomisator on versuchschemie.de has posted this same synthesis with many good
pictures.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|