| Pages:
1
2 |
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Assume the following system to get an indication of a possible implied net reaction (ignoring pH associated with speciation issues of active
transition metal ions):
H2S + H2O = H3O+ + HS-
Fe(lll)/Cu(ll) + HS- → Fe(ll)/Cu(l) + HS•
HS• + O2 → HSO2•
HSO2• → H+ + SO2•-
SO2•- + O2 → SO2 + O2•-
SO2 + H2O = H+ + HSO3-
HS• + O2•- → S + HO2-
HO2- + H+ → H2O2
Fe(ll)/Cu(l) + H2O2 + H+ --> Fe(lll)/Cu(ll) + OH• + H2O
OH• + H2S --> H2O + HS•
Approximate theoretical implied net reaction ignoring, not listed, problem side reactions (consuming radicals) and further oxidation of sulfite:
2 H2S + 2 O2 --Fe(ll)/Fe(lll)/Cu(l)/Cu(ll)--> S + H2O + H2SO3
------------------------------------------------------------------------------------
In addition to pH issues, transition metal(s) seemingly catalytic role and other possible catalyst (like chloride), chelates (like citric acid to
promote solubility), there are questions as to choice of particle size of say FeO.Fe2O3.
[Edited on 24-3-2018 by AJKOER]
|
|
|
Fulmen
International Hazard
    
Posts: 1749
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
I think you're following the wrong path:
https://pubs.acs.org/doi/abs/10.1021/jp980361p?journalCode=j...
"Initially, almost all the surface sites can interact readily with H2S, to form a layer of iron sulfides. Over longer reaction times reaction occurs
with deeper sites, to form bulk sulfides. Regeneration of ferric (hydr)oxide by oxidation with oxygen converts Fe2+ to Fe3+, and S2- anions are
replaced by O2- with formation of elemental sulfur"
So it's a two-stage reaction, the first is formation of iron sulfide in anoxic conditions. The second regeneration stage oxidizes the sulfide to
elemental sulfur.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Fulmen:
To quote from your source:
"The mechanism of the interaction of H2S with either reagent is ionic and involves heterolytic dissociation of H2S and exchange of S2- and SH- anions
for O2- or OH-. Subsequently water is eliminated and, in coupled redox reactions, sulfide is oxidized to elemental sulfur and Fe3+ cations are reduced
to Fe2+."
Dissociation: H2S + H2O = H3O+ + HS-
Coupled Redox reactions system:
Fe(lll)/Cu(ll) + HS- → Fe(ll)/Cu(l) + HS•
HS• + O2•- → S + HO2-
HO2- + H+ → H2O2
Fe(ll)/Cu(l) + H2O2 + H+ → Fe(lll)/Cu(ll) + OH• + H2O
OH• + H2S → H2O + HS•
Sulfide is oxidized to elemental sulfur:
.....
HS• + O2•- → S + HO2-
Fe3+ cations are reduced to Fe2+:
Fe(lll)/Cu(ll) + HS- → Fe(ll)/Cu(l) + HS•
Now, as to whether a 'layer of iron sulfides' is necessarily in place could be a function of complexing agents/chelates (like citric acid).
As to 'stages', note by my listing of reaction steps:
1. H2S + H2O = H3O+ + HS-
2. Fe(lll)/Cu(ll) + HS- → Fe(ll)/Cu(l) + HS•
3. HS• + O2 → HSO2•
.......
For a discussion of the surface chemistry on the action of H2S on Fe(lll), please see, for example, https://pubs.acs.org/doi/abs/10.1021/la00042a030 .
[Edited on 24-3-2018 by AJKOER]
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
I have deleted a long response...
I was unaware SEAPA discharge rules were changed, despite them sending me the paperwork i have been working to the wrong limits. Not sure it affects
anything written here but it does change what i need to do.
One thing i do need to mention, the discharge goes directly to feeding crops. the solid material (is hardly any) from anything organic goes onto
drying then pellets. I have to watch heavy metals ect which is kinda why i like Iron.
The scrubbers on large systems are pretty small, but they dont often handle seaweed, I also have another problem to deal with. But that can wait....
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Right we are cleaned out, I will autoclave tomorrow then reconnect. I got some different pumps to go on and a better PH probe (last one started
playing up).
Should be ready to fill Monday morning, will kick it off with cow shit again. Water jackets are connected in series and central reactor has a hot
plate with water jacket magnetic stirer, it also has internal stiring via outside motor. Two of the other have the internal system with outside motor
as well.
If I bump the temperature a little should it shouldnt take long to gas. I cant start with seaweed it dosnt seem to work well, i will put all my notes
online for download if anyone wants them.
Out of interest, any merit in using oxygen poor water to start it off? I use CO2 and H2 for flushing before i charge with the cow shit. Its collected
fresh....very fresh as in dosnt reach the floor fresh.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
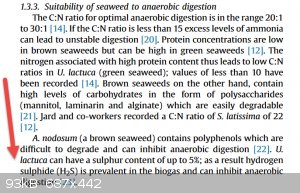
Found one reason i may be getting problems after storms etc.
|
|
|
| Pages:
1
2 |