aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Salting Out
A while back when NurdRage was having difficulty with his pyrimethamine Epic synthesis i stumbled across the compound vicine which has a similar
N-substituted pyridine ring structure.
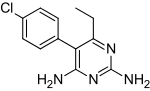
Pyrimethamine
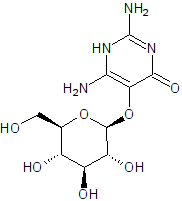
Vicine
The discussion was about extracting it from fava beans, lopping off the glycoside thingy and whacking on a chlorotoluene unit before shoving a bent
stick where the other O is.
My part was 'just' to find a way to suck vicine from the beans, with all the complex OC Nerdy's problem.
After some research it turns out that much work has been done on vicine & fava beans because it's aglycone divicine has been found to be the cause
of the disease 'favism' which affects some people if they eat those beans.
This paper is really good for getting vicine & convicine out of fava beans :-
Attachment: marquardt1983.pdf (2MB)
This file has been downloaded 464 times
Unfortunately the two stumbling blocks are an 'air classifier' which they use to separate the protein, fat & starch to create a protein
concentrate. Not got one.
The second is the use of a centrifuge producing up to 20k G. Not got one of those either.
Today i found a paper advocating the great benefits of 'salting out' in OC workups :-
https://pubs.acs.org/doi/full/10.1021/acs.oprd.7b00197
So, in amateur fashion i've de-hulled and mashed up some beans, added enough acetone/water/NaOH to match proportions as used in the paper, shoved it
in a cafetiere and used the plunger to remove most of the solids, added a pile of Na2SO4 then filtered a lot and adjusted the pH
to about 7 to see if anything happens.
One of several previous attempts achieved some crystals, although i have no idea what they were, nor any way of really knowing.
Now it appears that the paper recommends sodium sulphate, however my question is: how do you predict if your desired compound will get salted-out, or
salted-in ?
If any passing genius could answer this, i'd be grateful.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Anyone ?
https://www.youtube.com/watch?v=3ugSWeOntYE
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
The answer isn't as simple as some people think it is  But people sometimes
look at polarity of the desired compound and the ionic strength of the solution after the salt is added. I think ammonium acetate is a common choice
for amino acids. This isn't an amino acid, but I'd probably try that. But people sometimes
look at polarity of the desired compound and the ionic strength of the solution after the salt is added. I think ammonium acetate is a common choice
for amino acids. This isn't an amino acid, but I'd probably try that.
[Edited on 8-2-2018 by JJay]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Cheers JJay.
Part of my problem is not knowing how to work out if vicine is polar or non-polar.
Is there some way to know that just by the molecule's structure ?
[Edited on 8-2-2018 by aga]
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
I can tell by looking that vicine is polar as heck. All those hydroxyl groups and the amines are hydrogen bond donors, and the ketone and ether
groups hydrogen bond acceptors.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Whoah hoss !
Can you please point out to an OC noob what the hydroxyl and amine groups are.
I guess 'hydrox' means the HO bits and 'amine' means the NH bits.
Is that right ?
Which bit is a ketone ?
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
You are correct. Hydroxyl is -OH and amine is N with one to three carbons singly bound, with the difference made up by H. In this case, I meant the
primary amines (N with one carbon bound, -NH2) and the secondary anime (N with two carbons bound, -NH-). A ketone is O doubly bound to carbon, =O.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
That's really clear and very useful.
Thanks.
|
|
|