RogueRose
International Hazard
    
Posts: 1593
Registered: 16-6-2014
Member Is Offline
|
|
Red vs yellow PbO & some other Pb oxides
Took some lead acid batteries apart and ensured full oxidation of the PbO2 plates & seperated the “spongy” part from the mesh frames (for both
+ & - plates).
Tried melting some of the grey sponge with a blowtorch and it didn't melt into pure lead but formed yellow and melted red spots on the edges and
corners.
I powdered the brown plates and heated some of the powder with a blow torch which quickly turns yellow & forms red moulten nodules. The area just
below the surface seems difficult to heat with a torch so I ended up melting it all into a nice pool/bead of a dark grey that is very crumbly and has
yellow inside when broken/crushed. I then powdered the blob and got a fine yellow powder that seems darker than i've seen in pics as well as some
shiny specs similar to what sand looks like, in the powder. Could there be unrreacted pure lead mixed in giving it the darker than “normal”
appearance?
I also found that the only place I could get any pure Pb from the batteries was from melting the wire mesh frame and that only yeilded about 20-50%.
What is the easiest way to get PbO without using acids and then reducing them with heat? What is the difference between the red and yellow PbO, it
looks like the yellow is always formed after being melted so does it have a higher energy (electron – IDK what level Pb holds).
I was trying to make some PbO to use for making some salts as it seems that it would be much easier to make the salts with the oxide than with pure
Pb.
 
|
|
|
woelen
Super Administrator
        
Posts: 8014
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
There are different oxides of lead:
- PbO: A yellowish powder, not bright yellow, but quite close to what your picture shows. Some samples have a somewhat brownish color and would be
called beige instead of yellow. In dilute nitric acid, this oxide dissolves quite easily, giving a colorless solution of Pb(NO3)2.
- Pb3O4: A very bright orange solid. In dilute nitric acid this partially dissolves, giving a solution of Pb(NO3)2, leaving a brown solid behind,
which is PbO2 (see below).
- PbO2: A dark brown solid. This oxide is a strong oxidizer. It does not dissolve easily in nitric acid, but it can be dissolved in a large excess
amount of highly concentrated HCl, giving a bright yellow solution of PbCl4 (or PbCl6(2-)) in hydrochloric acid. On heating this liquid produces Cl2.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Just an additional note - Pb3O4 is actually a mixed valence oxide, 2PbO*PbO2.
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
last summer i found an old lead acid batter (it was huge so maybe from a truck) on a beach, i believe it had been there for quite a few years because
it was torn apart by the waves and i could see the exposed plates. anyway, i bring it home and dissassemble it compleatly. the lead plates were
heavily corroded, so by melting them i got maybe 5kg (from 20kg of battery). i didn't want to dispose of 15kg of lead oxide, i wanted the metal!
so i built a charcoal furnace out of a metal bucket and some plaster of paris. once the fire was lit i would add lead oxide and charcoal on the top,
and pump air from the bottom of the fire. i also should have used a flux of some kind (sodium carbonate was mentioned on the web) but i didn't have
any.
at the end (i stopped just because it was late...) i got at the bottom a solid lump of lead metal 
   
i plan to convert the other 10kg of lead oxide (plus a new battery i found) in the next future, maximum next month, so stay tuned for that 
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
That is an interesting question:
Indeed, there are two different modifications of PbO, the thermodynamically stable red Litharge and the meta-stable yellow Massicot. Because they do
not differ that much in their energy of formation (-219.1 kJ/mol vs -217.5 kJ/mol) it is possible to get the yellow one from the red one by heating it
to somewhat around 500°C. The process is reversible but quite slow, that's why you are able to get the yellow form. There seem to be other ways to
make it as well (I have never tried that myself but literature tells so). For example by careful decomposition of Lead(II)carbonate or Nitrate.
Heating the yellow modification for some time in water will form the red modification. The reason for this is, according to literature, that the red
modification is more insoluble in water and will leave the equilibrium of yellow <-> red that way.
It's not mentioned in my source here, but I'd guess you encountered something called Ostwald's rule. An interesting rule many people often forget
about. The first thing to crash out of a solution is usually the less stable one, so not the thermodynamic one, which explains, why you find many
colorful sulfides for example in nature, like cinnabar, but when you precipitate them on your own in the lab with H2S, you will always get
black samples. And the same things probably happen here. As you quench your reactions or you use methods where you precipitate the oxide, it will
first form the meta-stable one, and therefore you end up with the yellow modification.
Now about the structure, the Holleman-Wiberg explains that you have a square packing of oxide-anions with lead(II) being on top or below the square
plane forming pyramids....that is an interesting description but I don't like it...
Basically what you have is a CaF2-lattice, also called Fluorite lattice. It is a cubic structure where all tetrahedral sites are occupied.
As you may know there a two dominant way to stack crystals, in a hexagonal fashion and in a cubic fashion. For calcium fluoride, the calcium-cations
form a cubic lattice. These have the same amount of octahedral sites (which means octahedral holes, where you put in the counter-ion) as the packing
atoms and it has two times the amount of tetrahedral sites. The formula CaF2 already states, that you probably require twice the amount of
space for the fluoride than for the calcium. So it would be good to fill the tetrahedral sites and in a CaF2 all of them are filled.
Now I think to further explain the structure I'd have to use the antitype here, which is Li2O, it's the same thing but the roles of packing
and tetrahedral site-placement are switched. If you take this structure, you can 'thin' it out, which means, you occupy less and less tetrahedral
sites.
1/2 of them occupied would lead to PbO (litharge, red), CuO, PtS, ZnS (wurtzite and sphalerite) and many more. They differ in structure, depending on
what is required. But for PbO a layer-type structure is favored. That means you end up with layers and some space inbetween...why is this the case?
Well, you have a Pb(II), which means you still have a lone pair on the lead and this requires some space so you push it into the empty space between
the layers.
You can continue this thinning process to get to different related structures like ZnCl2, HgI2, SiS2 (those were all
1/4 occupied)....in the end you can even describe OsO4 in its crystalline solid form with a 1/8 occupation.
I think in the yellow modification the Pb will slip a bit and cause different bond lengths but I am not really sure, to be honest.
It's always best to visualize these things, so I also added you a small picture where I compared the different structures, I think I should have
chosen the Li2O-type to make it easier to see, sorry about that. I chose a 2x2x2 cell for all three structures, the color code is the same
for all, the metal being colorless-grey, the ion-sizes are rescaled so the anions have the same size in all three examples, the viewing direction is
always along -1, 0, 0, so that 0,1,0 points to the right, and 0,0,1, points downwards.
I am however not too certain about the massicot structure, that's the one I found on the Crystallography Open Database, which was published in 1961.
The lattices were visualized using Diamond 4.
EDIT: I also added the Li2O-type now
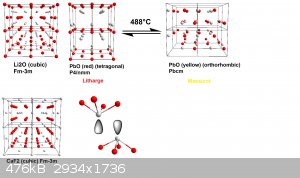
[Edited on 5-2-2018 by fluorescence]
|
|
|
RogueRose
International Hazard
    
Posts: 1593
Registered: 16-6-2014
Member Is Offline
|
|
I have about 20 test tubes going with lead from different states of the battery and seeing how they react with different acids, it is really
interesting as I would say about 1/2 don't behave as I thought they would and the temps for melting the oxides seems WWAAYY off (by like 500+F) and
reducing with carbon doesnt' seem to work and all kinds of other strange things which I had thought would be a fun project - but I suspect once I get
it figured out, then it will all be downhill from there.
Oh, as a note, that yellow PbO in my pics looks SOO much more yellow in this pic than it does in my house where it is much more dull and kind of
okra-ish. I think it is these damn CFL and LED light bulbs that they sell now. so many things look differently because of these light bulbs that it
really throws off some experiments now that I think of it, it seems that they are missing colors or they are never vivid.
|
|
|
|