PrussianBlue
Harmless

Posts: 38
Registered: 20-1-2018
Member Is Offline
Mood: No Mood
|
|
Para Sulfonation of Aniline
Would anyone happen to know the best way to para-sulfonate aniline? I'm supposed to draw up a synthesis of 2-bromo-4-chloro-nitrobenzene from benzene
and I'd like to be able to assure only ortho bromination of the aniline intermediate. Any thoughts?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2788
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
This paper probably has details:
http://pubs.acs.org/doi/abs/10.1021/ie50399a012
If you're not doing this for a class, you can probably obtain para-aminobenzoic acid and brominate that, followed by decarboxylation, which saves you
a few headaches.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
1.https://link.springer.com/article/10.1007/BF00957338
2.http://www.tandfonline.com/doi/full/10.1081/SCC-120014967
3.http://pubs.rsc.org/en/Content/ArticleLanding/1999/P1/a90153...
4.http://www.nrcresearchpress.com/doi/10.1139/v05-026#.Wm1lj7y...
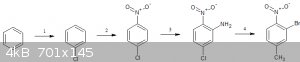
followed by oxidation and hunsdiecker,you mean 
[Edited on 28-1-2018 by CuReUS]
|
|
|
PrussianBlue
Harmless

Posts: 38
Registered: 20-1-2018
Member Is Offline
Mood: No Mood
|
|
Forgot to mention -- I am doing this for my Organic II class and I'm not allowed to use nucleophilic substitution for this one. Here's what I have
drawn up so far. Will the amine survive the two rounds of acid and heat?
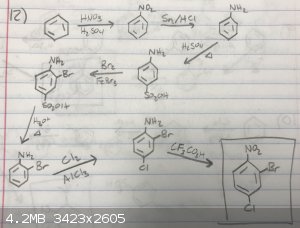
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
I don't see why not.The 2 things that needs to be changed is the omission of FeBr3 and AlCl3 in the bromination and chlorination
of aniline respectively,since aniline is very active to electrophilic substitution anyway and also the lewis acid would complex with the
NH2.I wonder if the desulphonation/chlorination could be done in one step ?
[Edited on 1-2-2018 by CuReUS]
|
|
|