Harristotle
Hazard to Others
  
Posts: 138
Registered: 30-10-2011
Location: Tinkerville
Member Is Offline
Mood: I tink therefore I am
|
|
Sources of choline chloride in Australia
Does anyone have a good cheap source of choline chloride in Australia? The chicken places here don't seem to sell it.
I am looking at playing around with a deep eutectic solvent and tlc. Several hundreg 6 to 1kg should do the job.
Cheers,
H.
|
|
|
j_sum1
Administrator
       
Posts: 6325
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
You could try health food places.
(Not that I have actually seen it but it is the best idea I could come up with. I don't have a "chicken place" nearby.)
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
As it happens i've been thinking of doing some DES experiments for ages, and got around buying the bits a few weeks (could be months) back.
Same situation here - no ChCl can be easily bought locally, then i remembered someone (blogfast? deltaH? dunno who) saying you can react choline
bitartrate with potatassium chloride in a simple metastasis reaction to get choline chloride and potassium bitartrate as the products.
Potassium bitartrate is grudgingly soluble in water which helps.
Choline bitartrate is sold as some kind of wonder-food, which is Good, as the purity is 99% (so it says on the label), and you can get it on Ebay.
Also good is that No-Salt Salt (KCl) is sold in the supermarket, although it needs recrystallising twice to get rid of the other junk,
So far i got white goop, but that is encouraging, so i'll do a utoob video on it IF it works as a DES along with the urea i already purified.

[Edited on 12-10-2017 by aga]
|
|
|
Harristotle
Hazard to Others
  
Posts: 138
Registered: 30-10-2011
Location: Tinkerville
Member Is Offline
Mood: I tink therefore I am
|
|
Thanks aga!
This is helpful. I shall do it that way then.
Cheers,
H.
|
|
|
NedsHead
Hazard to Others
  
Posts: 409
Registered: 9-12-2014
Location: South Australia
Member Is Offline
Mood: No Mood
|
|
Welcome back aga, glad to see you're back mate
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Having dissolved ChBit. and KCl, then mixed them, the KBit. drops out nicely, and in bulk.
Boiling down the ChCl solution looked like it was about to crystallise, so i put it in the fridge.
Turns out that the crystals are just more KBit.
The information i found that said that ChCl is 'reasonably soluble' is complete s**t. It is Very, if not Extremly soluble.
Next step will be filtering out the solids and then boil to dryness, probably take it all the way to melting at 302+ C and let it cool in some kind of
dessicator.
Maybe add some activated carbon before filtering to try to get rid of the yellow colour.
It's like CaCl2 all over again.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2736
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Choline will be very soluble, and likely hygroscopic, so it may not ever form a nice solid. Just used a derivative the other day, comes as the
calcium salt, as the sodium salt is harder to get solid.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
So far so good - i got pink stuff !

In the time it took to get that photo off the camera and resized, the pink turned to burnt 

Oh well. Either i can try to salvage the unburnt stuff, or start again.
|
|
|
G-Coupled
Hazard to Others
  
Posts: 287
Registered: 9-3-2017
Member Is Offline
Mood: Slightly triturated
|
|
What caused the charring - did you have a heat source on it, or was it just in a desiccator or similar?
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|
:(
I would not recommend drying chlorine chloride at a temp greater than 150 C. Today I had about 50g drying in an oven at 140(ish) C for 4 hours and had
a mostly dry powder. I decided to turn up the temp to 180 C in order to get it completely dry before storage. After leaving for about 10 minutes I
noticed a weird amine smell coming from the basement. I ran down and my white solid was turning brown, decomposing into some awful smelling products,
white vapors were seen as well. I can still smell the fishy smell in most of the house even after 30 minutes. Now I will have to repurify after the
hours and hours of boiling down/drying I already did.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Idiocy and messing with a camera instead of closely watching something happening on a hotplate at full whack caused the charring.
Redissolving, mixing with more activated charcoal, filtering again and boiling down again, but CAREFULLY this time, and it's back to a pink slush.

I think i'll stick it in a dessicator setup to see if it gets to be a powder that way.
Vaguely i imagine the burnt bits are contaminants, so allowing the charring to go to completion might have been a better idea.
We'll see.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Turns out that there's already a utoob video on making choline chloride from choline bitartrate by Tom's Lab, who i believe is a member here.
https://www.youtube.com/watch?v=5sbe0MpdWP0
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|
Yep, that's me. I added some narration to make the video a bit more bearable. Maybe it's up to aga's standards now 
https://www.youtube.com/watch?v=8P-ftOjfr40
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Much better video !
Shows the process very well indeed.
There's no Beer in the video, so my standards might not be quite met 
|
|
|
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
I also had problems with choline chloride so I replaced it with trimethylglycine, it took me a lot of time to figure this out, their structures are
similar, I also had success with making new eutectic solvents by using molecules that are similar to the more traditional eutectic solvents.
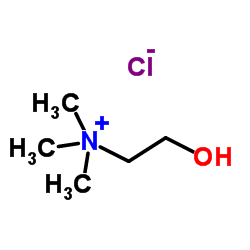
[Edited on 24-10-2017 by gatosgr]
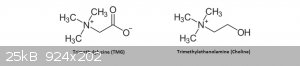
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by gatosgr  | | I also had problems with choline chloride so I replaced it with trimethylglycine, it took me a lot of time to figure this out, their structures are
similar, I also had success with making new eutectic solvents by using molecules that are similar to the more traditional eutectic solvents.
|
Wooo !
Sounds interesting. Please give more details.
|
|
|
Texium
Administrator
       
Posts: 4583
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by Geocachmaster  | | I would not recommend drying chlorine chloride at a temp greater than 150 C. Today I had about 50g drying in an oven at 140(ish) C for 4 hours and had
a mostly dry powder. I decided to turn up the temp to 180 C in order to get it completely dry before storage. After leaving for about 10 minutes I
noticed a weird amine smell coming from the basement. I ran down and my white solid was turning brown, decomposing into some awful smelling products,
white vapors were seen as well. I can still smell the fishy smell in most of the house even after 30 minutes. Now I will have to repurify after the
hours and hours of boiling down/drying I already did. |
Yeah back when I was messing with DES's I made that
same mistake, except instead of in a basement I was in a chemistry classroom full of students. Needless to say, they weren't too impressed by the
clouds of trimethylamine fumes and from then on dreaded my independent experiements.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by zts16  | | they weren't too impressed by the clouds of trimethylamine fumes and from then on dreaded my independent experiements. |

|
|
|
gatosgr
Hazard to Others
  
Posts: 237
Registered: 7-4-2015
Member Is Offline
Mood: No Mood
|
|
Check my signature.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Post id 403455 is permanently Engraved and held in high regard - it should be sticked or wikied or something.
What is lacking is an Application of that great information that you kindly provided.
I have a few ideas, yet have done nothing to test them, yet.
|
|
|