Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Synthesis of tetrahydrofuran derivative?
I was messing around for a while and thought about a possible synthesis (attachment). It seems fairly ridiculous and I doubt it would ever work
without a catalyst (more on this later). But here it is for R=Me:
Aldol condensation with acetone (deprotonated) + acetaldehyde gives pent-3-en-2-one, react with N-bromosuccinamide to yield the allyl bromide
derivative and hydrolyse with water to give A.
React A with 4-nitroaniline forming an imine to get that -M extended resonance ready.
Now the crazy bit.
React this with styrene in a cyclisation, forming a C-O and a C-C bond (enthalpically favourable!) and taking all the excess electrons via the
delocalisation. Sterics should only allow one structural isomer.
Proton transfer with the zwitterion gives the protected product, and upon hydrolysis of the amine gives the product
1-(4-phenyloxolan-3-yl)propan-2-one.
* if a catalyst is needed, the most plausible one would be one that binds to the alkene in styrene (or other alkenes for this reaction) and would be
electron deficient.
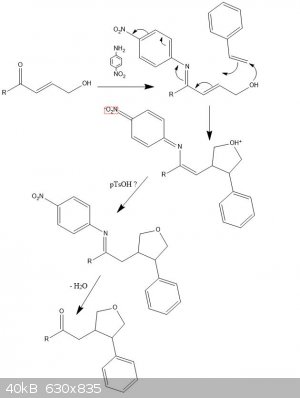
Edit: image should have been +H2O
[Edited on 15-3-2017 by Eddygp]
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Won't the aniline undergo a Michael reaction instead ?
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
True, conjugate addition is a fairly good competing reaction.
Nevertheless, will the following cyclisation take place? For that one, I can't see any competing reactions at all and it seems possible enough..?
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
clearly_not_atara
International Hazard
    
Posts: 2789
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
It looks like you are trying to create a 1,3-dipole? I don't think that what you have there is sufficient. Creating a C-C-O 1,3-dipole requires a lot
more electron-withdrawing stuff at oxygen; you need an oxonium ylide, basically, usually something that must be generated in situ. You can use an
excited epoxide which rearranges to a carbonyl ylide; that will result in a tetrahydrofuran derivative, but I don't know the specifics.
If anything you're looking at an aza-Diels-Alder cyclization between the unsaturated imine and styrene to a
1-(p-nitrophenyl)-2-phenyl-4-hydroxypiperidine.
|
|
|